 [Atkinsonia [Gaiadendreae [Elytrantheae [Psittacantheae + Lorantheae]]]]: leaves opposite; fruit viscid, rubber outside vascular bundles.
[Atkinsonia [Gaiadendreae [Elytrantheae [Psittacantheae + Lorantheae]]]]: leaves opposite; fruit viscid, rubber outside vascular bundles. EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. For further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
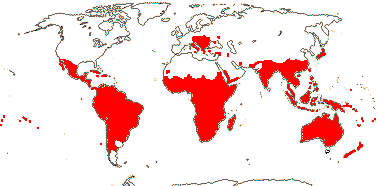
SANTALALES Berchtold & J. Presl - Main Tree.
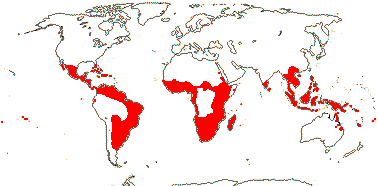
Mycorrhizae absent; acetylenic fatty acids [e.g. santalbic/ximenynic acid, triglycerides with C18 acetylenic acids], triterpenic sapogenins + [Loranthaceae?], essential oils; cork subepidermal; vessel elements with scalariform perforation plates [E]; perforation plates not bordered; intervascular pits alternate; axial parenchyma strands ³7 cells wide [E], rhombic crystals in ray cells [E]; tension wood?; nodes 3:3 [E]; pericyclic fibres 0; (cristarque cells +); petiole bundle annular [E], (cuticle waxes with annular rodlets, palmone common); petiole/mesophyll with (astro)sclereids; lamina margins entire; inflorescences cymose; flowers quite small [10> mm across], K small, open, cupular, teeth ± inconspicuous, C valvate, large and protecting bud, (apex inflexed ["hooded"]), hairs (long), erect, adaxial; A opposite C, anthers basifixed; pollen grains bipyramidal-spheroidal, surface smooth-perforate to reticulate; nectary [sometimes as "disc"] +; G [3], ovary septate below, placentation free central above, style +, stigma small; ovule 1/carpel, pendulous, apotropous, outer and inner integuments ca 4 cells across, micropyle endostomal, parietal tissue 0; embryo sac curved, with chalazal caecum; fruit a drupe, 1-seeded, K persistent; seed coat crushed/0; chalazal endosperm haustoria +, (endosperm with starch); embryo small/minute, chlorophyllous; plastome GC content 36-38%; germination hypogeal. - 14 families, 151 genera, 1,992 species.
Includes Aptandraceae, Balanophoraceae, Coulaceae, Erythropalaceae, Loranthaceae, Misodendraceae, "Mistletoes", Mystropetalaceae, Octoknemaceae, Olacaceae, Opiliaceae, Santalaceae, Schoepfiaceae, Strombosiaceae, Ximeniaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. Note that the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Anderson et al. (2005) date crown-group Santalales at 108-101 Ma.
Evolution: Divergence & Distribution. For the evolution of the hemiparasitic habit in Santalales, see below. Magallón and Sanderson (2001) described this as a very species-rich clade; Orobanchaceae include a comparable example. A [Mystropetalaceae + Loranthaceae] clade has been recovered in recent analyses (see below), which suggests that the holoparasitic habit has evolved twice.
Where a number of characters are to be placed on the tree is unclear. Those with an "[E]" after them in the characterization above are found in the first three families; if these form a single clade, the characterization will need to be adjusted accordingly. Grímsson et al. (2017a) summarized pollen variation in the clade, but see also Y. Yu et al. (2018) for pollen evolution.
Plant-Bacterial/Fungal Associations. Little is known about mycorrhizae in Santalales, but the few taxa studied largely lack them (Landis et al. 2002; see also Brundrett 2017b), exceptions being Ongokea, Coula and Strombosia (Malécot 2002 for references); the second two genera are not hemiparasitic. The absence of mycorrhizae, as well as that of root hairs in some taxa, is probably connected with the adoption of the hemiparasitic habit. The absence of root hairs is perhaps unlikely to be a synapomorphy for the whole clade (c.f. Judd & Olmstead 2004), and although there is little information on this feature in Kuijt et al. (2015), they are quite widely present in Santalaceae, at least (Fineran 1963), but I know nothing about root hairs in the first seven families below.
Genes & Genomes. The rate of change in the nuclear 18s rDNA gene has been greatly accelerated, but that in other nuclear protein-coding genes much less so (Su & Hu 2012). Nickrent et al. (2010: supplement) summarize information about chromosome numbers.
The evolution of the plastome in the order has been studied by X. Chen et al. (2019: Erythropalum scandens the only non-parasite included); there are genomic changes associated with the evolution of hemi- and holoparasitism.
S. Zhou et al. (2023: Fig. 1) discusses aspects of the evolution of the size, structure, etc. of the mitogenome in those few Santalales known. Intron loss via gene loss or merged exons and shift to trans-splicing is shown in the three members of Viscaceae + Santalaceae for which this is known, and there is nothing quite like it in the few other Santalales in the diagram; one looks forward to what better sampling may show. The mitochondrial coxII.i3 intron is absent in Comandra, in 2001 the only member of the order to have been sampled (Joly et al. 2001).
Chemistry, Morphology, etc.. For the general chemistry of the group, see Kubitzki (2015). For the distribution of the acetyleneic santalbic (= ximenyic) acid (E-11-octadecen-9-ynoic/octadeca-11-trans-en-9-ynoic acid) in this clade, see Aitzetmüller (2012); it is found in most groups, and Aitzetmüller (2012) suggests that there are similar compounds with quite wide distributions in the order. For (poly)acetylenic and related fatty acids in the seeds, see also Badami and Patil (1981).
Vascular pits are notably variously bordered throughout Santalales (Herendeen et al. 1999b); Carlquist (2006) suggests that non-bordered perforation plates are a possible similarity with Caryophyllales. The foliar vascular bundles may lack fibres (but c.f. Olacaceae, Loranthaceae, ?some Opiliaceae). Terminal veinlet tracheids and cristarque cells are scattered through the whole group (Baas et al. 1982; Kuijt & Lye 2005). Wax tubules with palmitone as the main wax occur in several members (Ditsch & Barthlott 1997).
The are a number of morphological issues surrounding the inflorescences and flowers. Nickrent et al. (2019) looked at inflorescence morphology throughout the order, focussing on the work of Suaza-Gaviria et al. (2017) and the distribution of cymose inflorescences; three nodes in the phylogeny were the focus of their discussion, but variation in infloresences elsewhere in the order was extreme and in groups like Santalaceae-Visceae and -Amphorogyneae they were simply very hard to understand. In the flower, there has been controversy over the nature of the various whorls encircling the flower. What appears to be the outer perianth whorl - often a minute, rim-like structure and sometimes without vasculature - has been interpreted as being a "caylculus" of paired, connate structures of prophyllar/bracteolar origin in a number of Santalales (Wanntorp & Ronse De Craene 2009; Ronse de Craene 2010; Ronse de Craene & Brockington 2013). However, if bracteolar, their shift on to the top of an inferior ovary needs explanation, as does their presence in the terminal flower of a cymule since these would not normally be expected to be immediately associated with any prophylls at all associated directly with them. Oddly, in Loranthaceae, a "calyculus" is described in flowers which are also shown as having a prophyll (Wanntorp & Ronse De Craene 2009). This "calyculus" seems best interpreted as a calyx that, initiated as a ring, may become irregularly lobed as it develops (Suaza Gaviria et al. 2016 and references). That being said, the calyx in Santalum and Loranthaceae like Struthanthus is unusual in that it initially does not completely encircle the flower, there being an interruption on the adaxial side. Furthermore, Johri and Bhatnagar (1971) noted that this structure is not regularly lobed and usually lacks any vascular supply, although it is vascularized in Nuytsia, at least (but not in most Loranthaceae). Finally, in Santalaceae like Comandra there is no evidence of any calyx, at least from gross morphology. It seems best to call any "calyculus", a calyx (see also Kuijt 2013, 2015; Robayo et al. 2020). The single perianth whorl of some Santalales may then represent the larger corolla/inner perianth whorl of other members (Wanntorp & Ronse De Craene 2009; Ronse de Craene & Brockington 2013; Kuijt 2015), however, at least sometimes its members are reported to have three vascular traces, two coming from commissural bundles (F. H. Smith & Smith 1943). The vasculature of the inferior ovary of Darbya (= Nestronia, Santalaceae) and other members of the order - there are recurrent bundles in the gynoecium - suggests that it has become inferior by investment of tissues that are axial in origin, although Exocarpus and some other genera have a superior ovary (F. H. Smith & Smith 1942, 1943; Eyde 1975).
Another issue concerns the interpretation of the complex embryology in the order, or even within the single family, Balanophoraceae. The ovule, embryo sac and embryo development of many plants in this clade all all more or less remarkable. This is evident in the absence/loss of the integuments and the disappearance of an organized ovule and placental tissue in many taxa; the number of integuments in "Olacaceae" is unclear (e.g. Johri & Bhatnagar 1960; Maas et al. 1992; Breteler et al. 1996; Malécot 2002; summary in R. H. Brown et al. 2010 and references). Distinct integuments, or even distinct ovules, may not be recognizable, the embryo sacs being borne in a spherical body, the mamelon; this may consist of a basal placenta containing embryo sacs (see e.g. Paliwal 1956; Ross & Sumner 2005; R. H. Brown et al. 2010 for discussion as to what this structure might actually be). For information on genes expressed during ovule/embryo sac development, see Brown et al. (2010). Brown et al. (2010) surveyed the distribution of integument number across the order, and looked at the expression of two genes, one expressed during integument development in Arabidopsis and the other in the chalaza; they found that both genes were expressed in the tissues immediately surrounding the embryo sac and in the wall surrounding the loculus of ovules in Santalaceae-Santaleae and -Visceae that apparently lacked integuments. This suggested either that the integument(s) had become fused with the nucellus or that gene expression had shifted (see also Gasser & Skinner 2017).
Tracheids in the "nucellus" have been reported from some species, as has vascular tissue directly reaching the embryo sac (Werker 1997). Some taxa may have both micropylar endosperm and embryo sac haustoria (Mickesell 1990), and variation in embryo and endosperm development and embryo sac morphology is very considerable (see e.g. Johri & Bhatnagar 1960; Kuijt 2015), and the remarkable embryo sacs growing up the style of Loranthaceae are without parallel elsewhere (see Bachelier & Friedman 2011 for literature). There may sometimes be two ovules per carpel (see Maas et al. 1992). Further developmental studies are much needed.
The embryo sacs are often much elongated, even approaching the stigma at the end of the long style, as in several Loranthaceae. Ross and Sumner (2005: Arceuthobium) and York (1913: Dendrophthora) describe the antipodal end of the embryo sac as being apical on the mamelon, while Guignard (1885: Osyris) and Zaki and Kuijt (1994: Viscum) show it as being basal. There seems to be variation here in the growth of the ?chalazal end of the embryo sac (see also Rutishauser 1935; L. N. Rao 1942b, etc.), and York (1913) even described the chalazal ends of the two embryo sacs of Dendrophthora as fusing. Bhatnagar and Agarwal (1961) draw an embryo sac of Thesium in the apical prolongation of the nucellus; might it be the prolongation of a normally-positioned embryo sac?
Luna et al. (2017) suggest that the large nectary lobes alternating with the corolla in Jodina (Santalaceae) and the corolla lobes themselves develop into the fleshy part of the fruit, the latter eventually falling off and the former persisting and forming the flesh of the drupaceous fruit; the stone is mesocarpial in origin. Endosperm development and the endosperm haustorium are variable, the latter sometimes being shorter than antipodal haustorim (L. N. Rao 1942b); the endosperm haustorium may demolishe first the placenta and integument and finally the endocarp, furthermore, the endosperm has nests of tracheary cells in it (Luna et al. 2017: Fig. 5C, D). Antidaphne seems to have three vascular traces in its cotyledons.
Corolla hairs commonly occur in small tufts immediately abaxial to where the stamens are inserted on the petals, as in Strombosia and most Santalaceae. The anther wall is monocotyledonous in development in Maburea (Erythropalaceae: see Maas et al. 1992). It is difficult to estimate the thickness of the single integument - even when you think there is one - because it is often more or less digested by the endosperm quite soon after fertilization (e.g. Bhatnagar & Agarwal 1961).
For general information, see van Tieghem (1896), Kuijt (2015), the Parasitic Plants website (Nickrent 1998 onwards), Heide-Jørgensen (2008) and Nickrent (2020), for the first six families in particular, the old Olacaceae, see Reed (1955) and Malécot et al. (2004), both general, Baas et al. (1982: leaf anatomy), Sleumer (1984a: New World taxa, pollen, anatomy, etc., 1984b: Malesian taxa, general), Lobreau-Callen (1980, 1982: pollen) and Johri (1962: embryo sac). For other information, see Roberston (1982; Olac).
Phylogeny. For information on relationships, see Kuijt (1968: olacacean complex central), Nickrent and Duff (1996) and Nickrent et al. (1998). Malécot (2002) analyzed the variation in four genes emphasizing members of the old Olacaceae; he discussed variation of morphological characters in the context of molecular and combined morphological-molecular phylogenies. A number of clades appear to be fairly well supported. Erythropalaceae s.l., including Strombosiaceae and Coulaceae (Coulaceae were not included in all analyses, and their position was rather labile) are perhaps sister to all other Santalales and are free-living (Malécot 2002). These clades tend to differ in most probably plesiomorphic features (e.g. life style) from other Santalales (data from Michaud 1966; Malécot 2002; Malécot et al. 2004: a morphological analysis). However, exactly where they should be placed on the tree still waits for a strongly-supported resolution of relationships within Santalales, indeed, Coulaceae were not immediately associated with Erythropalaceae and Strombosiaceae in any analyses (Su et al. 2015). Malécot et al. (2004) found some support for Erythropalaceae, Ximenia plus some other genera, and most of the rest of the old Olacaceae (a very weakly supported clade) as three clades successively sister to the rest of Olacales in a morphological analysis. Molecular data place Octoknema rather differently than do morphological observations, which put the genus with the free-living members of Santalales (e.g. Malécot et al. 2004); if the latter position is confirmed, this may suggest that there have been a number of reversals in habit in this clade. Malécot and Nickrent (2008) have since found that the old Olacaceae formed about eight clades basal to other Santalales, but relationships between these clades were unclear (Nickrent et al. 2010) and remain so in the analyses of Su et al. (2015). Few members of these basal clades were included by Z.-D. Chen et al. (2016). Resolution was further improved by Nickrent et al. (2019: nuclear + chloropast genes, no holoparasitic taxa), who obtained the relationships [Erythropalaceae [Strombosiaceae [Coulaceae [[Ximeniaceae [Aptandraceae + Olacaceae]] [Octoknemaceae (support for this position very poor) + parasitic taxa]]]]]. The phylogeny of the whole family is summarized by Nickrent (2020) in a tree that was a combination of Nickrent et al. (2019), Su et al. (2015) and unpublished data on Octoknemaceae, although no details on how the combination was done are given. There relationships among the non-parasitic taxa are [Erythropalaceae [[Octoknemaceae + Strombosiaceae] [Coulaceae [[Ximeniaceae [Olacaceae + Aptandraceae]] ...]]]].
The big picture of relationships between the hemiparasitic taxa may be stabilizing somewhat (see Su et al. 2015, esp. Nickrent 2020). Within the Loranthaceae et al. clade, Misodendraceae are often sister to [Schoepfiaceae + Loranthaceae], especially in analyses that include many taxa, although when the number of taxa is reduced they may be sister to Schoepfia in particular (Malécot 2002, see also Nickrent et al. 1998; especially Der & Nickrent 2008; Vidal-Russell & Nickrent 2008; Nickrent et al. 2010; Su et al. 2015: both plastid and non-plastid genes); this topology is followed here. Opiliaceae (one genus) were strongly supported as being sister to a clade [Santalaceae + Viscaceae] in Soltis et al. (2007a; see also Nickrent et al. 2010). Der and Nickrent (2008) thought that Santalaceae were polyphyletic, a few genera being placed in Opiliaceae and Schoepfiaceae, while within Santalaceae there are eight well supported clades, although some with unclear relationships. Z.-D. Chen et al. (2016) obtained some support for a paraphyletic Santalaceae that included Opiliaceae. Relationships in Nickrent et al. (2019, see also Nickrent 2020) are [[Loranthaceae [Misodendraceae + Schoepfiaceae]] [Opiliaceae + Santalaceae]]; H.-T. Li et al. (2021) also found this grouping, and with good support, although relationships in much of the rest of the order could be better supported and Balanophoraceae s.l. were not included. Relationships in the order as suggested by the Seed Plant Tree, Angiosperms353 nuclear genes as of ix.2024 and by Zuntini et al. (2024) suggest some problems ahead. The former suggest [Sarcophyte [most other Balanophoraceae [[[[[Octoknema + four more clades of Olacaceae [Schoepfiaceae [Misodendraceae] [[Opiliaceae + Santalaceae (inc. Geocaulon)] [Mystropetalaceae + Loranthaceae]]]]]]]]]]]. The latter suggest [Maburea trinerva [other Olacaceae [Balanophoraceae [[[Misodendraceae + Schoepfiaceae] [Mystropetalaceae + Loranthaceae]] [[Geocaulon [Santalaceae + Opiliaceae]]]]]]] - the "other Olacaceae" include genera from three of the clades in the Seed Plant Tree, Maburea represents a fourth. Interestingly, a [Mystropetalaceae + Loranthaceae] clade was recovered in both analyses.
Balanophoraceae s.l. (= Balanophoraceae s. str. and Mystropetalaceae) are the only holoparasitic Santalales, and determining their relationships has posed some problems. Since they are holoparasitic, they lack most or all of the distinctive vegetative and even floral features of other Santalales (for holoparasitism, see Cai 2023). However, they do usually have only a single ovule per flower and that ovule has a curved, bisporic embryo sac (e.g. Fagerlind 1945c, d); the first feature might suggest relationships to Loranthaceae in particular. Balanophoraceae have granule-containing tracheidal tissue, known also from Loranthaceae, Opiliaceae, and throughout Santalaceae - and also Orobanchaceae (H. C. Weber 1986). A phylogeny of Balanophoraceae (Nickrent 1998: accessed 16.5.2009) based on nuclear SSU rDNA data suggested that [Mystropetalon [Dactylanthus + Hachettea]] (see clade B below) were sister to a clade containing the rest of the family (clade A below). These three genera also formed a clade (100% p.p.) that was sister to a clade made up of Schoepfia, Dendrophthoe and Santalum (almost 100%), the combined group having 100% support (Nickrent et al. 2005). Although Nickrent et al. (2005) suggested that Balanophoraceae were to be placed within Santalales, not sister to them, the former position had very little support. Su and Hu (2008, 2011) analysing variation in B-class floral genes and with a quite good taxon sampling suggested that Balanophoraceae were basal or near basal in the clade since they found the euAP3 homologue in Balanophora, but not in other Santalales. Su and Hu (2012) looked at several mostly nuclear genes; relationships were still not clear, but Balanophoraceae certainly seemed to be outside Santalaceae. Su et al. (2015) attempted to clarify the situation in their analyses, which included 11 species of Balanophoraceae and 186 other Santalales. Balanophoraceae formed two clades in maximum likelihood analyses, one (A) being sister to [Loranthaceae [Schoepfiaceae + Misodendraceae]] [Opiliaceae + Santalaceae]], while the other (B) was placed within the [Loranthaceae [Schoepfiaceae + Misodendraceae]] clade, while in strict maximum parsimony analyses both were in this latter clade and perhaps forming a monophyletic group; branch lengths in clade A were very long. In the plastome analysis of X. Chen et al. (2019) those Balanophoraceae included, members of clade A, were placed [Loranthaceae [Schoepfiaceae + Balanophoraceae]]. See also Nickrent and Duff (1996), Nickrent (2002) and Barkman et al. (2007).
Recently, Nickrent (2020) provided a summary of relationships and has again suggested that Balanophoraceae clades A and B were not sister taxa, relationships in this part of Santalales being shown as [clade A [[[Misodendraceae + Schoepfiaceae] [clade B + Loranthaceae]] [Opiliaceae + Santalaceae]]]. Hence the two clades are placed separately below, clade A being Balanophoraceae sensu stricto and clade B being Mystropetalaceae. However, the isssue is surely not settled. Ceriotti et al. (2021) recovered a clade A Balanophoraceae whose placement was uncertain (see Ceriotti 2021, also under Balanophoraceae below, although sampling there was slight. Mystropetalaceae/clade B were not included. The i.2022 version of the Seed Plant Tree of Life suggests the following relationships - [Octoknemaceae [some Balanophoraceae [Erythropalaceae [Strombosiaceae [[Coulaceae + more Balanophoraceae] [[Erythopalaceae, Aptandraceae, Olacaceae] [Ximeniaceae [[Opiliaceae (inc. Anthobolus) + Santalaceae] [Schoepfiaceae [Misodendraceae [Balanophoraceae - Hachettea + Loranthaceae]]]]]]]]]]]], albeit some nodes are not well supported. In another version of the sequence is [Olacaceae [Balanophoraceae *[Mystropetalaceae [[Opiliaceae + Santalaceae] [Loranthaceae *[Misodendraceae + Schhoepfiaceae]]]]]], but a number of families were not included. Given the preceding, and from the discussion of family limits in Cheek et al. (2024), an order-wide study using nuclear data might be helpful - Loranthaceae and Santalaceae s.l. can probably be represented by placeholders.
Classification. For a classification of all Santalales except Balanophoraceae, see Nickrent et al. (2010). The seven small families for the seven clades of the poorly-supported basal pectinations are recognised pending resolution of relationships there; if any are sister taxa, they probably could be combined. Of course, if the basal pectinations were not recognised as families, the whole order would then have to be placed in a single family (c.f. Su et al. 2015: p. 500). Families within the old Santalaceae are not recognised, despite the inclusion of the highly autapomorphic ex-Viscaceae there. Note that the classification of Santalales in Kuijt (2015) tends to follow morpology in part, and Erythropalaceae are not included at all, although three of the four genera mentioned below as being in that family are placed in a broadly circumscribed Olacaceae. "Mistletoes" are an ecological grouping made up mostly of aerial ectoparasitic Santalales - see below.
Previous Relationships. Santalales have often been compared with Icacinaceae (now known to be polyphyletic). Both have a single-seeded fruits, often small calyx, valvate corolla, etc. (e.g. Takhtajan 1997). However, there is little other evidence for such a relationship; for Icacinaceae, see especially Aquifoliales, Icacinales and Metteniusales.
Thanks. I thank M. F. Braby for information on pierine host-plant preferences.

Synonymy: Anthobolales Dumortier, Balanophorales Dumortier, Erythropalales van Tieghem, Heisteriales van Tieghem, Loranthales Link, Olacales Martius, Osyridales Link, Viscales Berchtold & J. Presl, Ximeniales van Tieghem - Balanophoranae Reveal, Santalanae Reveal - Loranthopsida Bartling, Santalopsida Brongniart
ERYTHROPALACEAE Miquel, nom. cons. —— Synonymy: Heisteriaceae van Tieghem - Back to Santalales
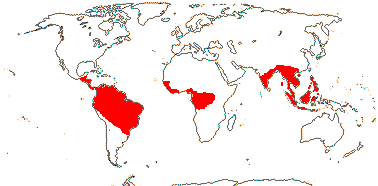
Trees, shrubs, or lianes with branch tendrils; (gallic acid +); laticifers +/0; (vessel elements with simple perforation plates); ground tissue of fibre tracheids; sieve tubes with non-dispersive protein bodies; (nodes 5:5); (epidermal/stomatal cells lignified; with druses), stomata various, cuticular thickening +, large guard cell chamber; leaves spiral or two-ranked, lamina vernation conduplicate, (venation palmate), (petiole pulvinate, margin toothed); inflorescence fasciculate or cymose; flowers (medium-sized), (4-6-merous); K ± free/connate basally; C connate to free, (adaxial hairs 0); stamens = and opposite K or C (with two lateral scales), 2X C; (pollen tricolporoidate); (disc 0); G 10-ridged, ± inferior [Erythopalum], opposite sepals (opposite petals) or odd member adaxial, (not septate), style short, stigma ± lobed or not; ovules with micropyle exostomal, (unitegmic); fruit 5-valved [Erythropalum], K much accrescent, fleshy, spreading, lobed or not [Heisteria]/not; endosperm with starch or oil, (cotyledons orbicular, foliaceous); n = 16, x = ?
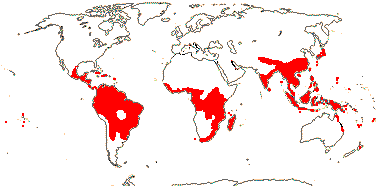
4/40 [list]: Heisteria (30). Pantropical, East Malesia to Talaud and Flores, not Madagascar or East Malesia and to the S.E.; most in Central and South America. Map: from Sleumer (1984a, b), Malécot (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010). [Photo - Flower, Fruit.]
Chemistry, Morphology, etc.. The laticifers of Heisteria are both articulated and non-articulated (Baas et al. 1982).
The stamens in an individual flower differ quite considerably in size, and the smallest stamens are opposite the petals, the largest stamens opposite the sepals (Michaud 1966). For some information on pollen, see Lobreau-Callen (1982). The gynoecium is often 10-ridged. A nectary is sometimes present, being described as adnate to the ovary (Sleumer 1984a) or on top of the ovary (Sleumer 1984b; c.f. Nickrent et al. 2010).
Baas et al. (1982: they examined Brachynema ramiflorum) recorded only infrequent and thin-walled sclereids. However, in the material examined here (see below) there were numerous sclereid nests in the cortex, indeed, they sometimes formed an almost a continuous layer outside the pericycle. Furthermore, stem, petiole, and also, judging from the way young leaves had dried, even the midrib have strongly sclerified diaphragms in the pith; the inside of the xylem cylinder was strongly fluted. Sleumer (1984a, q.v. for stamen position, etc.) described the inflorescence as being an ebracteate corymb. The seed coat is almost obliterated, and it is difficult to make out details of cell walls. Sleumer (1984) described the endosperm as having amylum and fatty substances; the endosperm stains rather weakly for starch, and the cells contain yellowish globules, the "fatty" and "sticky" substances below.
See Kuijt (2015) for some general information (esp. under Olacaceae) and Maas et al. (1992) for information on Maburea.
The embryology of this clade is unknown.
Phylogeny. Molecular data place Brachynema, a genus that is so morphologically distinctive that its inclusion in the order was in some doubt (e.g. see versions 7 of this site and earlier), close to Maburea, in Erythropalaceae s. str. (K. Wurdack, pers. comm.; Nickrent et al. 2016, 2019; not mentioned by Nickrent et al. 2010). Nodal anatomy and stomatal morphology, at least, are in agreement with this position. Maas et al. (1992) noted the similarity of Maburea and Brachynema in leaf anatomy.
Previous Relationships. Brachynema has often been associated with "Olacaceae" s.l., thus Lobreau-Callen (1980) placed it in Anacoloseae (= Aptandraceae here) and Baas et al. (1982) placed it with Scorodocarpus (= Strombosiaceae) in particular (see also above). It has also been linked with Ebenaceae (Ericales), as by Reed (1955) and others, while in a morphological phylogenetic analysis it appeared close to Symplocaceae (Ericales: Malécot 2002).
[Strombosiaceae [Coulaceae [[Ximeniaceae [Aptandraceae + Olacaceae]] [Octoknemaceae [Balanophoraceae [[[Mystropetalaceae + Loranthaceae] [Opiliaceae + Santalaceae]]]]]]: C ± connate; A adnate to C; embryo sac elongated, often curved, with a chalazal caecum, micropylar caecum +, ± developed.
STROMBOSIACEAE van Tieghem —— Synonymy: Scorodocarpaceae van Tieghem, Tetrastylidiaceae van Tieghem - Back to Santalales
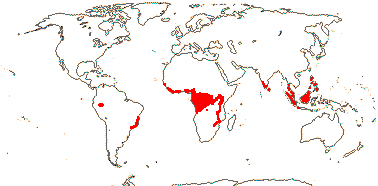
(Mycorrhizae +); ?santalbic acid; (vessel elements with simple perforation plates); ground tissue of libriform fibres; (nodes 1:1, 5:5); (petiole bundle with adaxial bundles); (groups of minute unlignified fibres associated with foliar vascular bundles); epidermal cells crystalliferous, with silica sand, stomata aniso-, cylco-, (helico)cytic; leaves spiral to 2-ranked, (lamina venation palmate); inflorescence fasciculate; flowers 4-5 merous, (hypanthium +); C (fleshy), (adaxial hairs 0); (nectary extrastaminal - Engogemona); A & adnate to C, = and opposite, (10, adnate on either side of C - Scorodocarpus), (filaments short, connective massive, anthers transversely multiseptate - Tetrastylidium), (loculi dehiscing separately - Engomegoma); tapetal cells bi/multinucleate; pollen tricolpate/tricolporoidate; G [3-6], (inferior), style short to long; ovules (5), (unitegmic, integument ca 6 cells across); (megaspore mother cells several), (embryo sac caecum 0); endosperm starchy, chalazal endosperm haustorium unicellular, growing into the funicle, embryo tiny; n = 20.
6 [list]/18. Scattered Pantropical (map: Sleumer 1984a, b; Malécot 2002; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010). Photo - Fruit.]
Evolution: Ecology & Physiology. Strombosia pustulata is one of the 18 species in the 8 Central African rainforests examined that together made up 50% of the above-ground biomass (at 1.8%, in 6 of the 8 sites: Bastin et al. 2015).
Chemistry, Morphology, etc.. For general information, see Sleumer (1984a, b) and Kuijt (2015: as Olacaceae, no Diogoa), Agarwal (1961a, 1963b) for Strombosia, and Breteler et al. (1996) for Engomegoma.
Phylogeny. Scorodocarpus and Strombosia are successively sister to the rest of the family (Nickrent et al. 2019).
Age. The age of this clade is about 103.6 Ma (Magallón et al. 2015).
COULACEAE van Tieghem - Back to Santalales
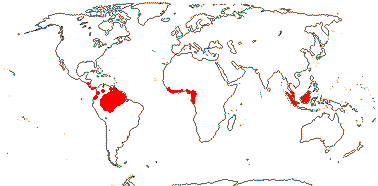
(Mycorrhizae +); ?santalbic acid; laticifers +; mesophyll ?lignified; epidermis lignified, with druses, epidermis with cork-warts [from stomatal complexes]; hairs dendritic; lamina venation scalariform; inflorescence (branched), with 3-flowered cymes along axis; flowers sessile; C basally connate, (adaxial hairs 0); A 10-20; pollen grains tricolporoidate, large verrucae along colpi and in polar regions; nectary 0?; G [(3-)4(-5)], style 0-short, stigma ± lobed; ovules with outer integument 5-6 cells across, inner integument 5-6 cells across; endosperm with starch; n = x = ?
3 [list]/3. Interrupted pantropical (map: Sleumer 1984a, b; Malécot 2002; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010).
Evolution: Ecology & Physiology. Found in 2 of the 8 Central African rainforests sampled, Coula edulis is described as a hyperdominant species (Bastin et al. 2015: one of the 18 trees that made up 50% of the above-ground biomass - it makes up 2.7%, number three on the list).
Chemistry, Morphology, etc.. The arrangement of the androecium is complex (Kuijt 2015).
For general information, see Sleumer (1984a, b) and Kuijt (2015).
Phylogeny. Relationships are [Ochanostachys [Minquartia + Coula]] (Nickrent et al. 2019).
[Ximeniaceae [Aptandraceae, Olacaceae [Octoknemaceae [Balanophoraceae [[[Mystropetalaceae + Loranthaceae] [Misodendraceae + Schoepfiaceae]] [Opiliaceae + Santalaceae]]]]]: root hemiparasites [contact with host by xylem]; vessel elements with simple perforation plates; axial parenchyma strands 7³ cells wide; nodes 1:1; sclerenchyma fibres of petiole and median vein often 0; petiole bundle arcuate; silicification of mesophyll cells +, cuticular thickening +, guard cell chamber small; plastome with ≤80 genes, NADH dehydrogenase complex 0.
Age. Moore et al. (2010: 95% highest posterior density) suggested ages of (99-)96(-91) Ma for this clade, 98-75 Ma is the age in H.-J. Su et al. (2015), while around 128 Ma is the age in Z. Wu et al. (2014).
Evolution: Divergence & Distribution. Santalales are somewhat unusual in that they contain a sizeable hemi/holoparasitic clade that makes up the bulk of the order and is much more diverse than its free-living sister groups. Thus the successive sister taxa to this hemi/holoparasitic clade have only 3, 18 and 40 species respectively. This is the reverse of the size relationships between holoparasitic and their sister non-parasitic clades (Hardy & Cook 2012). Orobanchaceae include a clade made up of hemi- and holoparasites that is similar in size, and again, the first three non-parasitic clades have around 12, 7 and 6 species respectively (see below).
Aerial hemiparasitism has been derived from root hemiparasitism some five times (Nickrent 2002; Watson 2020), with a few intermediate taxa being both root and stem parasites (Vidal-Russell & Nickrent 2008). Aerial hemiparasites evolved between the mid-Cretaceous to the early Eocene 75-55 Ma (Watson 2020 for a summary), well before the evolution of the birds and mammals that now disperse their seeds. However, Watson (2020) suggests that mammals were early involved in the transition from root to stem parasitism, carrying seeds up into the trees. Marsupials may have been involved in the case of the red-fruited Loranthaceae, and transport may have been endozoic, and primates in the case of the white-fruited Visceae, transport there being ectozoic, the seeds sticking to the animal's fur and being removed as it groomed (Watson 2020).
XimSanEcPhyEndress (2011a) thought that the inferior ovary in Santalales might be a key innovation for them. However, it is difficult to assign ovary position to a particular place on the tree. Many taxa in the families above have a superior ovary, and so do, for example, [Exocarpos + Omphalomeria], a basal clade in Santalaceae (Der & Nickrent 2005), Loranthaceae are inferior, Schoepfiaceae are half inferior, and more or less superior ovaries are also found in the clade. Either there are independent origins for the character of inferior ovary, or reversals, or both.
Individual embryo sacs may elongate greatly and approach the apex of the mamelon or even the stigma at the end of a long style. Haig (1990) suggested that this may allow competition between female gametes given that their normal spatial constraints (i.e., being enclosed in an organised ovule, see below) are absent.
Ecology & Physiology. The plesiomorphic life style in Santalales is to be free-living, and hemiparasitism by attachment to the roots of the host is derived, but just once (Malécot 2002; Malécot et al. 2004; Nickrent et al. 2010); for hemiparasitism, see also Fineran (1991). Aerial hemiparasitism has been derived some five times (Nickrent 2002; Watson 2020), with a few intermediate taxa being both root and stem parasites (Vidal-Russell & Nickrent 2008); photosynthesis in some aerial hemiparasites occurs largely in their stems. Aerial hemiparasites may have a single point of attachment to their host, or roots running over the surface of the bark may form both additional points of attachment and additional plantlets (as in Loranthaceae: Vidal-Russell & Nickrent 2006, 2008; Mathiasen et al. 2008: good survey of stem parasites). Some aerial parasitic Santalales are largely endophytic, although the part of the plant that is visible is chlorophyllous (Santalaceae, e.g. some species of Viscum and Arceuthobium), while Mystropetalaceae and Balanophoraceae are holoparasitic (Nickrent et al. 2005; see also Tesitel 2016). A few Santalaceae in particular are hyperparasites, often parasitizing Loranthaceae, and some species of Phoradendron (Santalaceae) and Globimetula (Loranthaceae) are even obligate parasites on other members of the same genus (Calvin & Wilson 2009; C. A. Wilson & Calvin 2016: "epiparasites"; Krasytlenko et al. 2022). For general information on parasitism, see Kuijt (2015), for haustorial anatomy, see papers cited by H. C. Weber (1984) and for physiological details of parasitism, see Stewart and Press (1990).
Genes & Genomes. X. Chen et al. (2019) examined plastome evolution in this clade. They found that various genes had been lost, several of which could be placed at particular nodes of the tree (e.g. the NADH dehydrogenase complex above), but drastic changes were restricted to the two Balanophoraceae. There was a relaxation of selection on both photosynthetic and housekeeping genes with the transition from root to stem parasitism (Chen et al. 2019). For plastid evolution in parasitic flowering plants in general, see Wicke and Naumann (2018), and in particular Santalaceae and Balanophoraceae below.
Chemistry, Morphology, etc.. Gran(ul)iferous tracheary elements are found in the haustoria of several unrelated members of the clade; the granules are usually proteinaceous, but are made up of starch in Ximenia (Fineran & Ingerfeld 1982). The haustoria may vary considerably in morphology (Kuijt 1968), but I have not followed this up.
Cocucci (1983) outlined variation in ovary morphology and the distribution of starch-containing tissues (primarily in the style or the mamelon); these latter may be involved in the extraordinary growth of the embryo sac. In some taxa the endosperm surrounding the single developing embryo is a composite affair, representing the products of more than one embryo sac (Fagerlind 1947a, 1948; Maheshwari 1950; Bhatnagar & Johri 1960; Ram 1970; Bhatnagar 1970; Bhandari & Vohra 1983; Johri et al. 1992; Shamrov et al. 2001; Subrahmanyam et al. 2015; Kuijt 2015 and references). However, more work on embryology and gynoecial morphology is needed. For seedlings, see Whigham et al. (2008 and references).
Phylogeny. Details of lamina anatomy largely agree with the circumscription of this clade (Baas et al. 1982).
XIMENIACEAE Horaninow - Back to Santalales
(Axillary thorns - Ximenia); rhombic crystals (and silica bodies) in ray cells; ground tissue of fibre tracheids; (stomata anomocytic); (lamina venation palmate); inflorescence ± umbellate; C 4, 5, 8, 10, free; A (= and opposite C), 2 (3) x C, (adnate to C), (filaments very long - Curupira); anther wall with only one middle layer, tapetal cells uninucleate; nectary 0; G superior, style short to long; ovules (unitegmic/ategmic), "strikingly linear"; cotyledons connate or not; n = 12 (13), x = ?; germination hypogeal.
4 [list]/13: Ximenia (10). Pantropical, warm temperate. Map: from van Balgooy (1993), Fl. Austral. vol. 8 (1984), Sleumer (1984a, b), Malécot (2002)) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
Chemistry, Morphology, etc.. Ximenia americana is crassinucellate. Some information is taken from Sleumer (1984a, b) and Kuijt (2015), all general, see also Sankara Rao and Shivaramiah (1978: embryology).
Phylogeny. Relationships here are [Malania [Ximenia + Curupira]] (Nickrent et al. 2019).
[Aptandraceae, Olacaceae [Octoknemaceae [Balanophoraceae [[[Mystropetalaceae + Loranthaceae] [Misodendraceae + Schoepfiaceae]] [Opiliaceae + Santalaceae]]]]]: pollen grains ± oblate; ovules anatropous, unitegmic or ategmic; endosperm digesting inner part of fruit wall [?Apt.].
Age. An approximate age for this clade, if it exists, is 96.6 Ma (Magallón et al. 2015: no Aptandraceae).
Phylogeny. There is no strong evidence that this is a clade; I have simply optimized some characters here.
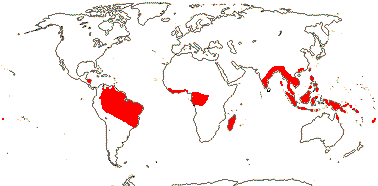
APTANDRACEAE Miers —— Synonymy: Cathedraceae van Tieghem, Chaunochitonaceae van Tieghem, Harmandiaceae van Tieghem - Back to Santalales
(Liane, recurved hooks [?nature] - Keita), (root suckering - K.), branches plagiotropic [?many]; (arbuscular mycorrhizae + - Ongokea); benzaldehyde + (0 - Anacolosa), laticifers +; rhombic crystals in ray cells; nodes 1:1, 1:5; (petiole bundle fibres +); (epidermis with cork-warts [from stomatal complexes] - some Aptandra); twigs somewhat zig-zag; leaves 2-ranked, (petiole articulated - K.); (plant dioecious); flowers 4-6 merous, bracteoles ± connate; (K minute), C ± free (connate half way), (with apical thickenings), (adaxially glabrous); A connate around style, epipetalous/free, (extrorse), (anthers valvate - 3-valvate in Hondurodendron), (connective prolonged - K.), (filaments short); pollen grains mostly oblate, (heteropolar), (4-porate, quadrangular in polar view), (6-aperturate, tri-diporate); (nectary 0 / outside A [A.] / alternating with A); G [2(-3)], (style deeply impressed, stigma cpitate - K.), stigma lobed; ovules often bitegmic; fruit surrounded by fleshy, ± unlobed, accrescent disc, K, or adjacent structures, (not K.), (nut-like); (seed-coat longitudinally invaginated - K.), (endosperm with starch), cotyledons 0-2, connate or not; n = x = ?14
9 [list]/35: Anacolosa (15). Pantropical (also S.E. China, Formosa). Map: from Michaud (1966), van Balgooy (1993), Sleumer (1984a, b), Malécot (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010). [Photo - Flower.]
Age. For records of the distinctive breviaxial tri-diporate fossil pollen Anacalosidites, very similar to pollen of Anacalosa, Cathedra, and Phanerodiscus, from the Upper Cretaceous (Europe, Australia: Maastrichtian, 75-65.5 Ma) and Palaeocene and particularly Eocene (worldwide) onwards, see Krutzsch (1988), Malécot (2002), Malécot and Lobreau-Callen (2005), and Carpenter et al. (2015).
Chemistry, Morphology, etc.. The hooks of Keita are described as being petiolar (Cheek et al. 2023), but their nature is unclear - no mention of prophyll position, etc..
In the anthers of Chaunochiton (Aptandra clade) each loculus opens by a separate slit. Androecial variation in Aptandraceae is considerable, as is palynological variation; pollen grains that have four apertures tend to be quadrangular in polar view (Grímsson et al. 2017a). The fruits of Phanerodiscus have what appear to be five, small, backwardly-pointing green perianth lobes, and opposite these are five large, strongly lobed fleshy structures that loosely surround the fruit.
Some information is taken from Sleumer (1984a, b) and Kuijt (2015), all general; see also Ulloa Ulloa et al. (2010) for the remarkable Hondurodendron.
Phylogeny. There are two easily-characterizable clades within Aptandraceae. The Aptandra clade, with five genera, includes taxa with a more or less extra-staminal nectary, valvate anthers, pollen with concave meso- and apocolpium, and a calyx that is accrescent in fruit, while the Anacalosa clade, with three genera, has lignified guard cells (unique in Santalales), anthers dehiscing by pores and with prolonged connectives, diploporate pollen, and the disc or extradiscal area is accrescent in fruit (Malécot et al. 2004; Nickrent et al. 2010, 2019). Some taxa in both clades have petals with apical thickenings. Cheek et al. (2024) recently described the distinctive climber, Keita (see above); where exactly it is to go remains to be seen.
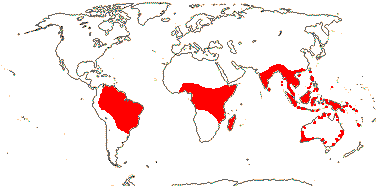
OLACACEAE R. Brown, nom. cons. - Back to Santalales
SiO2 bodies in ray cells; (nodes 1:3); leaves 2-ranked; flowers 3-6-merous, (heterostylous); (K 0); (C 3 - Olax [?connate in pairs]); A 2-3 x C, staminodes +/0, (paired), opposite K, thecae long; tapetal cells 2-4-nucleate; pollen grains (tricellular), 3-porate, (tri-diporate); G ridged or not; ovules ategmic, (unitegmic, integument 5-6 cells across), (nucellar cap + - Olax); embryo sac (bisporic [chalazal dyad], eight-celled [Allium-type]), elongated, (growing to the base of the style); K much accrescent/not; endosperm (chalazal haustorium 4-nucleate), (growing into pedicel), starch slight, embryo tiny, cotyledons 0-1; n = 12, x = 7 (?6), nuclear genome [1 C] (0.06-)1.745(-51.064) pg.
3 [list]/57: Olax (40). Pantropical (also southern China, Formosa). Map: from Sleumer (1984a, b), Fl. Austral. vol. 8 (1984), Malécot (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
Evolution: Ecology & Physiology. For the hemiparasitism of Olax phyllanthi, see Tennakoon et al. (1997 and references); the haustoria tap the xylem and can take up quite substantial amounts of carbon from the host (Tesitel et al. 2010c). Hibberd and Jaeschke (2001) provide a model of nutrient flow between host and parasite.
Chemistry, Morphology, etc.. Dulacia is heterostylous. Again the smaller stamens may be opposite the petals, the larger stamens opposite the sepals. The embryology of Olax is known, and it is quite variable.
Information is taken from Sleumer (1984a, b) and Kuijt (2015), all general; for embryology, see Shamanna (1954) and Agarwal (1963a).
Phylogeny. For the relationships [Ptychopetalum [Olax + Dulacia]], see Nickrent et al. (2019).
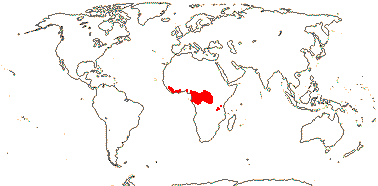
OCTOKNEMACEAE van Tieghem nom. cons. - Back to Santalales
?Parasites; ?santalbic acid; essential oils 0; axial parenchyma slight, strands ³4 cells wide; phloem with bundles of fibres; nodes 5:5; sclereids/cristarque cells +; petiole bundle annular (with medullary bundles); silicification of mesophyll cells 0; hairs stellate or dendritic; stomata cyclocytic, anomocytic, etc., cork warts on leaf [from hair bases]; plant dioecious; inflorescences branched or not, in fascicles; (flowers 3-merous); C adaxially glabrous/papillate; staminate flowers: pollen ± tricolporoidate; (disc +); pistillode; carpelate flowers: staminodes +; (glands alternating with staminodes); G [3 (5)], inferior, stigma 3-lobed, lobes bifid/flap-like, multi-lobed; integuments 2 or 1; seed longitudinally ruminate [(6) 9-11 lamellae], radicle relatively very long, cotyledons 6; n = x = ?
1 [list]/14. Tropical Africa (map: from Gosline & Malécot 2012; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010).
Chemistry, Morphology, etc.. For a summary of what is known about this genus, see Gosline and Malécot (2012), also Kuijt (2015).
[Balanophoraceae [[[Mystropetalaceae + Loranthaceae] [Misodendraceae + Schoepfiaceae]] [Opiliaceae + Santalaceae]]]]: stem and/or leaves often with transversely-oriented stomata; inflorescence axis indeterminate; (bracteoles 0); G not septate, style hollow; ovules undifferentiated, ategmic; embryo sac ± protruding at the micropyle-growing up stylar canal; endosperm digesting inner part of fruit wall; testa 0; chalazal endosperm haustorium with single nucleus; chloroplast ndh genes 0.
Age. Naumann et al. (2013) estimated the age of a clade [Balanophoraceae [Loranthaceae + Schoepfiaceae]], the only Santalales in the study, at ca 92.5 Ma (the ages in Table 2 are for a different node); given the findings of Su et al. (2015), this clade, or something similar, may hold. The node [[Loranthaceae [Misodendraceae + Schoepfiaceae]] [Opiliaceae + Santalaceae]] has been dated to (102-)97, 85(-80) Ma by Wikström et al. (2001), (115-)99, 91(-76) Ma by Bell et al. (2010) and ca 90.6 Ma by Magallón and Castillo (2009).
BALANOPHORACEAE Richard, nom. cons. - Back to Santalales
Root parasites, echlorophyllous; ± tuber-like; santalbic acid?, lignans +, plant tanniniferous; roots 0; extensive endophytic growth, rhizome or tuber-like structures either parasite or parasite-host mixed, these rupture as the infloresence develops and leave a collar-like structure at ground level; stems/inflorescences endogenous; cork ?; vessel elements with simple perforation plates; cuticle wax crystalloids 0; leaves spiral, two-ranked, whorled, or 0; stomata 0; plant monoecious or dioecious; inflorescence ± capitate or spicate, terminal, axis racemose, inflorescence bracts peltate or clavate, vascularized, subtending fascicles of flowers; flowers minute, (slightly monosymmetric); staminate flowers: bracts peltate-clavate or 0; P 0, 3-4(-8), valvate (imbricate), free (basally connate); A equal and opposite P, (1-2, esp. when P = 0), extrorse, usu. connate, (thecae connate), dehiscence irregular; (endothecium biseriate); pollen grain surface ± rugulate; pistillode 0 (+); carpelate flowers: P 0 or minute; staminodes 0; G 1, [2, 3 (-5)], inferior [?all], 0-3-locular, styluli +, impressed/style single, stigma punctate or ± capitate; ovary solid [mamelon visible early], "ovules" 1-2, reduced to an embryo sac, ?polarity, pseudoendothelium +/0, vascular supply 0; embryo sac (chalazal caecum 0), polar cells divide, or central cell coenocytic; bracts accrescent, fleshy; fruits minute, (nut-like), 1-seeded; seed coat 0; endosperm cellular, (diploid), chalazal haustorium single-celled, embryo undifferentiated, 4-12 cells, suspensor 0; plastome 15.1-28.4 kb, ≤27 genes, GC content ≤21.2%; mitogenome multichromosomal [?family].
14/41: [list: 5 subfamilies]. Pantropical. Map: from Hansen (1972, 1980, 1986) van Balgooy (1975), Heide-Jørgensen (2008) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5. (2010). [Photo - Flower]
1. Sarcophytoideae Engler —— Synonymy: Sarcophytaceae Kerner
Inflorescence branched; pollen triporate; carpellate flowers sunk in receptacle; stigma sessile.
2/2. Africa.
[Balanophoroideae [Scybalioideae [Lophophytoideae + Helosidoideae]]]: ?
2. Balanophoroideae Engler —— Synonymy: Langsdorffiaceae Pilger
Rhizome containing balanophorin [wax]; vascular bundles chimaeric [vessel-transfer cell-vessel]; leaves large; inflorescence apex develops inside lysigenous + schizogenous cavity [B.]; bracts accrescent, fleshy [= spadicle, claviform body - B.]; staminate flowers: P uniseriate, whorled/spiral; pollen 3-5 zonoporate (8-12-pantoporate/inaperturate - B.), quadrangular in polar view and surface echinate [but hardly - L.]; carpelate flowers: embryo sac Polygonum-type; endosperm few-celled/copious [L.] plastome colinear with that of other angiosperms, cis-spicing introns 0, trnE pseudogene, non-functional; germinating seed attaches to host by sticky protrusions of endosperm cells [all B.]; n = ca 16, ca 18; plastome TAG/TGA [stop codons] code for tryptophan.
3/30: Balanophora (24). Pantropical.
[Scybalioideae [Lophophytoideae + Helosidoideae]]: ?
3. Scybalioideae Engler - Scybalium Schott & Endlicher —— Synonymy: Scybaliaceae A. Kerner
Bracts triangular; hairs dense, filiform; pollen spheroid, 6-8-pantopororate, tri-/bicellular, surface granular; fruit achenial.
1/4. Greater Antilles and South America.
[Lophophytoideae + Helosidoideae]: plastomes colinear, but not with those of other angiosperms, loss of rpl14, rps 2, 11, etc., genes, trnE 0.
4. Lophophytoideae Engler —— Synonymy: Lophophytaceae Bromhead
Underground parts starchy; vessel elements of host and parasite in contact ["simple" haustoria]; runners +, apical meristem +, root cap 0, epidermis + [Om., La.]; tapetal cells uninucleate [Lo.]; pollen 3-6-colporate (3-colpate), (surface verrucate); G 2, stigmas capitate, placentation axile; ovules 2, ategmic, vascular tissue 0; embryo sac inverted, tetrasporic, 8-celled [Adoxa type] [Lo.]; plastome TAG/TGA [stop codons] code for tryptophan.
3/12: Ombrophytum (7), Lophophytum (4). South America.
5. Helosidoideae van Tieghem —— Synonymy: Helosidaceae Bromhead.
Rhizome with starch [Roph.]; vessel elements of host and parasite in contact ["simple" haustoria]; inflorescence ± scapose, inflorescence bracts multiseriate filariae [He.]; pollen 3-colpate, usu. tricellular, triangular-convex-triangular in polar view [last two]; embryo sac bisporic [chalazal cells], 4-celled, central cell huge, antipodal cells 0 [Helosis-type] / bisporic [chalazal spores], 8-celled [Allium type] [Cor.]; endosperm multicellular [He.], plane of first cleavage of zygote horizontal [He.], embryo few-celled [He.].
4/6: Helosis (3). Mexico and Central and South America, Madagascar, the Himalayas to S. China and Malesia.
Evolution: Ecology & Physiology. Families of plants parasitized by Thonningia sanguinea (Balanophoroideae) are all laticiferous (references in Quintero et al. 2016); little is known about how the host is infected. Balanophora abbreviata attaches to the host by sticky endosperm tubules, tubular primary haustorial embryonal processes then infecting the host (Arekal & Shivamurthy 1976); the whole process looks like infection by a T4 phage. Lambio et al. (2023) found that Balanophora abbreviata in the Philippines (Luzon) grew inside caves, perhaps on Ficus roots.
There has been very extensive movement of host mitochondrial genes into Lophophytum and Ombrophytum, both Lophophytoideae; this is discussed under Genes & Genomes below.
Pollination Biology & Seed Dispersal. Birds, small mammals and insects have been recorded visiting the flowers of Balanophoraceae, and the nectar can be quite copious (Santos et al. 2016). Amorim et al. (2019) found that opossums visited the flowers of Scybalium fungiforme in the Atlantic Forest of S.E. Brazil, nectar produced by extrafloral nectaries on the inflorescence being an attractant, and hummingbirds and especially bees and wasps were also visitors. Quintero et al. (2016) thought that Thonningia sanguinea in Uganda was pollinated by sunbirds; they noted that plants of different sexes were over 2 km apart on average, which does make one wonder somewhat. The inflorescences of Langsdorffia and Thonningia are pseudanthia (Baczynski & Claßen-Bockhoff 2023). A variety of insects have been recorded as visiting Balanophora (Lambio et al. 2023). Sato and Gonzalez (2017) and Gonzalez et al. (2019) suggested that there was parthenogenesis in Lophophytum and Helosis respectively.
Birds may disperse the fruits of Balanophora yakushimensis, being attracted by the red, fleshy, accrescent bracts, and other animals may also be involved in fruit dispersal in this genus (Suetsugu 2020).
Vegetative Variation. In Balanophoraceae s.l., the vegetative body is very highly modified; as Pellissari et al. (2022: p. 331) noted, "nearly all Balanophoraceae species lack proper roots, stems, leaves, apical meristems and a true epidermis, and even stomata could be entirely absent".
Genes & Genomes. Schelkunov et al. (2020/2021) looked at the transcriptomes of two Balanophoraceae, Balanophora and Rhopalocnemis. They were quite similar, both showing extensive loss in genes involved in photosynthesis and quite substantial gains in some other classes of genes, perhaps particularly those involved in various aspects of nucleic acid metabolism. The A-T content of the genome was unremarkable, as was that of other photosynthetic Santalales. X. Chen et al. (2023) compared the genome of Balanophora with that of Sapria (Rafflesiaceae), and in some respects they were quite similar - parallelism.
Incorporation of information about plastome and mitogenome variation into the characterisations above has been helped by the review of those genomes by Sanchez-Puerta et al. (2023). The plastomes of non-photosynthetic plants are in general A-T rich, but those of Balanophoraceae known are extremely A-T rich, at least 85.2% A-T, perhaps because of the loss of genes involved in plastid genome replication, recombination and repair (Su et al. 2019; Schelkunov et al. 2019, 2020/2021; Ceriotti et al. 2021: Balanophora and Rhopalocnemis phalloides). The plastomes are very small, about one tenth the size and much modified compared with those of autotrophic plants, and this might be expected. For example, the two species of Balanophora examined have only 15 genes, and about a third of these overlap, intergenic spacers are small, and all tRNAs needed for plastid protein synthesis must be imported (see also Pilostyles - Apodanthaceae). There have even been changes in the genetic code. Thus in Balanophora, at least, TAG is no long a stop codon, but codes for tryptophan (Su et al. 2019: ?Thonningia) while in Rhopalocnemis TAG is not used for anything (Schelkunov et al. 2019). Furthermore, in both Lophophytum and Ombrophytum TGA (also a stop codon elsewhere) and TGG both code for tryptophan (Ceriotti et al. 2021, q.v. for how this might happen). The pattern of gene loss/transition to pseudogene of trnE is also interesting (Ceriotti et al. 2021). Substitution rates are also high in the plastome of Balanophoraceae s.l. (Schelkunov et al. 2020/2021).
There has been very extensive movement of host (Fabaceae, esp. Mimoseae) mitochondrial genes into Lophophytum mirabile (Lophophytoideae) - horizontal gene transport (HGT) is involved - and, as in Amborella, it has been suggested that mitochondrial fusion may have been involved (G. Petersen et al. 2020 and references). About 60% of the mitogenome sequence is from Fabaceae, of which 49% is from mimosoids in particular, and the mimosoid genes are ca 80% of those of the parasite mitochondrion and are apparently functional (Sanchez-Puerta et al. 2016, 2018, 2023; see also Petersen et al. 2020). There are also host nuclear-encoded subunits forming part of the mitochondrial complex, and these subunits have remained very largely unchanged - just one gene has been replaced (Gatica-Soria et al. 2021). The enzyme complexes such as the oxidative phosphorylation and the cytochrome c maturation systems that are now coded for by this mosaic mix of genes remain functional. Interestingly, the respiratory capacity of parasitic organisms is sometimes reduced, but apparently not here (Santos et al. 2018; Gatica-Soria 2021 and references). Roulet et al. (2020) looked at the mitogenome of Ombrophytum subterraneum, also Lophophytoideae, and found that 34/51 of the circular chromosomes contained genes, 12 of these genes (and sometimes almost entire chromosomes) coming from Asteraceae, Fabaceae and Lamiaceae in particular, but other families were also donors; some of the legume sequences were shared with Lophophytum. The mitochondrial genes cox1, atp1 and matR of Ombrophytum showed massive divergence (Barkman et al. 2007). Interestingly, in taxa like Ropalocnemis there has been no HGT (Sanchez-Puerta et al. 2023).
S. Zhou et al. (2023) discuss the evolution of numerous chromosomes in the mitogenome of Thonningia sanguinea, 924 unique repeats were detected, and all chromosomes shared a 896-bp repeat.
Chemistry, Morphology, etc.. Langsdorffia, Thonningia and Balanophora have balanophorin, a wax-like substance, rather than starch as the main reserve.
For the anatomy of the vegetative body of Lophophytum, see Gonzalez and Mauseth (2010).
It is difficult to understand the morphology of the flower, i.e the number of stamens, whether the ovary is superior or inferior, the basic morphology of the ovule, the curvature of the embryo sac, fruit type, etc.. For the initial development of the flower of Balanophora, see Shivamurthy et al. (1981). Pollen variation in Balanophoraceae s.l. is quite extensive (Hansen 1980, 2015; summary in Grímsson et al. 2017a). Note details of pollen morphology in Hansen (2015) and Grímsson et al. (2017a). Holzapfel (2001) provided a critical summary of the variation in the nucellus, embryo sac, etc.. Eberwein et al. (2009) note that the carpellate flowers of Balanophora may entirely lack any appendages and be adnate to clavate bodies, perhaps modified bracts, that are borne in no particular order on the inflorescence. Balanophora has a mamelon-type structure rather unlike that of other members of the family (Teriokhin & Yakolev 1967). For discussion as to whether or not female flowers of Balanophoraceae s. str. have a perianth, see Gonzalez et al. (2019). Fagerlind (e.g. 1945c) noted that the "apical" cell of the megaspore tetrad in Langsdorffia develops into the embryo sac, but other than describing the position of the cell in the embryo sac relative to the inflorescence axis determination of what is apex and base seems to be impossible. There may be reversed polarity of the embryo sac in Balanophora and Lophophytum, at least (Arekal & Shivamurthy 1978; Sato & Gonzalez 2016). Embryo sac development varies, and Gonzalez et al. (2019: Table 1) summarize the variation in a number of gynoecial features in Balanophoraceae s.l. and a few related Santalales. In Balanophora, Lophophytum and Langsdorffia, at least, there is a ?chalazal caecum, as in many other Santalales, but any such caecum is weakly developed in Dactylanthus and absent in Corynaea (Johri et al. 1992; Holzapfel 2001; Sato & Gonzalez 2016). Holzapfel (2001) noted that a pseudoendothelium was quite common in the family.
Endosperm development is sometimes described as being helobial. The basal cell produced by the first division of the endosperm nucleus is massive and the nucleus usually remains undivided (if it does divide, cell walls do not form - Ernst 1914); it is perhaps to be compared with the chalazal haustorium in other Santalales. Division of the smaller upper cell and its descendants always involves the formation of cell walls (see also Ekambaram & Panje 1935). Dahlgren (1923) called the endosperm cellular; see also Gonzalez et al. (2019) for Helosis, where the central cell of the 4-nucleate embryo sac is huge. But this all seems simple compared with some reports. In the formation of endosperm, a coenocytic structure with 2-11 nuclei first forms, the cells coming from the antipodal region, there is also incorporation of nuclear and cytoplasmic material from the nucellus, then all the nuclei, including some from the nucellus, fuse to form a single meganucleus, and then that nucleus divides, and when there are ca 12 nuclei, cell walls form...
In Helosis the inner layer of cells of the fruit wall is massively thickened on the inner and anticlinal walls, very unlike that of other Santalales, while in Balanophora it is the outer layer that is thickened. Since an ovule cannot be distinguished, describing the fruit other than vaguely is difficult; the fruits are often described as being achenes or nots. Gonzalez et al. (2019) described the first division of the embryo in Helosis as being horizontal.
For additional information, see Hooker (1859), van Tieghem (1896, 1907), Kuijt (1969), Takhtajan (1988, 1997), the Parasitic Plants website (Nickrent 1998 onwards), Heide-Jørgensen (2008), Nickrent 2020 and Hansen (1980: Neotropical taxa, 2015, but written some time before: worldwide), all general; see also Mauseth et al. (1992: Ombrophytum), Pellissari et al. (2022: esp. Lathrophytum peckoltii) both vegetative, Hansen and Engell (1978: inflorescence morphology); Hansen (1976: pollen nucleus number; stigma surface), Solms-Laubach (1867) for haustorial anatomy, Zweifel (1939), Harms (1935b), Fagerlind (1938a, 1945b, c, d) and Sato and Gonzalez (2013, 2016, 2017 - Lophophytum), all embryology, and Baskin and Baskin (2021) for seeds, etc..
Phylogeny. As mentioned, [Mystropetalon [Dactylanthus + Hachettea]] are sister to a clade containing the other Balanophoraceae (Nickrent 1998: accessed 16.5.2009; Su et al. (2012: unrooted tree) and Su et al. (2015), however, other details of relationships within Balanophoraceae s.l. are unclear (see also discussion above). Ceriotti et al. (2021) found the relationships [[Balanophora + Thonningia] [[Lophophytum + Ombrophytum] [Rhopalocnemis, Corynaea, Helosis]]], the relationships of Sarcophytum within the clade being unclear. Some features of plastome variation seem to be congruent with this topology.
Classification. Given Balanophoraceae s.l., even s. str., what about other families that might be recognized? Kuijt (1968) placed all the families that have been segregated from Balanophoraceae and Mystropetalaceae (apart from Hachetteaceae) as separate families in his Balanophorales. However, Hansen (2015) recognized a broadly circumscribed Balanophoraceae that included seven subfamilies, of which Mystropetaloideae and Dactylanthoideae make up Mystropetalaceae here. Su et al. (2015; see also Nickrent 2020) thought that Balanophoraceae were polyphyletic, and included Mystropetalon, Dactylanthus and Hachettia in Mystropetalaceae, the other genera were included in Balanophoraceae s. str. - that classification is followed here. For a subfamilial classification of Balanophoraceae s. str., see Hansen (2015) and Sanchez-Puerta et al. (2023).
Previous Relationships. Cronquist (1968) thought that Balanophoraceae and Santalales were related, and later (Cronquist 1981) he placed them all in Santalales. However, Kuijt (1968: p. 138) thought that any connection between Balanophoraceae and Santalales s. str was "an historical accident and taxonomic artefact". In the past Balanophoraceae have often been much more widely circumscribed since parasitic plants show common adaptations to the parasitic habitat and their flowers are often very much reduced, so at one level, they all look similar. Thus Takhtajan (1997) linked Balanophoraceae with Cynomoriaceae (Saxifragales), Rafflesiales (here Malpighiales) and Hydnoraceae (here Piperales), relationships rejected by Kuijt (1968), who included them all in his Magnoliidae.
Botanical Trivia. Balanophora has the smallest flowers of any angiosperm; female flowers may be made up of a mere 50 cells, and there may be one million flowers per inflorescence - yet the inflorescences are just a few centimetres tall (Su et al. 2019 and references).
[[[Mystropetalaceae + Loranthaceae] [Misodendraceae + Schoepfiaceae]] [Opiliaceae + Santalaceae]]: (transverse stomata +).
Age. This node has been dated to (102-)97, 85(-80) Ma by Wikström et al. (2001); Bell et al. (2010) suggested an age of (115-)99, 91(-76) Ma and Magallón and Castillo (2009) suggest ages of ca 90.6 Ma, Naumann et al. (2013) estimated that its age was around 67.1 Ma, while an age of around 143 Ma can be obtained from Maul et al. (2018: Fig. 2)...
Genes & Genomes. Shin and Lee (2018) looked at the chloroplast genomes of eleven species of hemiparasitic Santalales; ndh genes were lacking in all of them (see also Mower et al. 2021 for possible connections between various distinctive life styles, including parasitism, that might affect the photosynthetic process and result in the loss of such genes). Shin and Lee (2018) also observed a number of gene losses specific to particular species or groups of species.
Chemistry, Morphology, etc.. Stems have tranversely orientated stomata in Visceae and Loranthaceae, at least (Kuijt 1959), indeed, such stomata on stem and/or leaf are scattered throughout the fdamilies that follow, perhaps most notably common in Visceae, although there has been no exhaustive survey of their distribution (see also Butterfass 1987; Wilson & Calvin 2003; Rudall 2023b). Furthermore, this clade is notable in that a number of species lack much in the way of a periderm or cork cambium, however, there is something called a cuticular epithelium. This lacks the radial files of cells that characterize a cork cambium, it has no lenticels, and lacks much in the way of suberin, rather, it has successive cuticular layers (Wilson & Calvin 2003). This distinctive structure seems to occur in a subset of the taxa with horizontally arranged stomata, i.e., in a number of Santalaceae, see Chemistry, Morphology, etc..
For inflorescence morphology - which can be hideously complex - in this group of families, see Suaza-Gaviria et al. (2017); the main axis seems to be indeterminate, the lateral branches more or less modified cymes with a fair amount of "fusion"/recaulescence, etc.. Suaza-Gaviria et al. (2017) rightly note that inflorescences here are not racemes.
For general information, see Calder and Bernhardt (1983).
But first, a note about MISTLETOES. In ecological literature in particular "mistletoes" often implicitly or explicitly include members of Loranthaceae, Santalaceae-Visceae, --Amphorogyneae and/or -Santaleae in particular, and also Misodendraceae, i.e. they make an ecological grouping (rather like mangroves) that includes aerial ectoparasites for the most part (see discussion and references in Watson 2001; Aukema 2003; Ndagurwa et al. 2016; Krasylenko et al. 2021; Watson et al. 2022). The groups just mentioned should be consulted for more on the particularities of ecology and physiology, pollination and seed dispersal, plant-animal interactions, etc., that they show. Furthermore, what goes on in (hemi)parasitic Orobanchaceae may be of interest; root parasitism is the norm there.
Evolution: Divergence & Distribution. D. M. Watson et al. (2022) suggest that being an aerial parasite dispersed by birds was a key innovation driving diversity in mistletoes. For details of seed dispersal, see below.
Ecology & Physiology. As background: Hemiparasites in general tend to have high concentrations of nutrients in their leaves (Krasylenko et al. 2021). The water use efficiency of mistletoes is lower than that of their hosts because they have a lower photosynthetic capacity and higher transpiration rates; chlorophyll contents of mistletoes are lower than those of their hosts (e.g. Cocoletzi et al. 2020). Thus Glatzow and Geils (2009) found that Loranthus europaeus kept on transpiring after the stomata of its host, Quercus robur, had closed, indeed, its own stomata remained open after it started to wilt itself. Indeed, the parasite grew better even as its host grew more poorly - it was less shaded. Comparing the deciduous and evergreen hosts of Psittacanthus schiedianus, Cocoletzi et al. (2020) found that the parasite affected the photosynthesis of both, and also their nutritional status, and that this was most noticeable in the evergreen host, and they noted that others had also found that the photosynthetic rate of the parasite was affected by the N concentration of its host. Y.-B. Zhang et al. (2023: most species examined Loranthaceae) found evidence for the nitrogen parasitism hypothesis, that is, low water use efficiencies and high transpiration rates of the parasite are mechanisms for the mistletoe to acquire adequate nitrogen. They found that P and K concentrations in the parasite were higher than in the host, those of N similar or slightly lower.
Loranthaceae and Santalaceae in particular can have considerable effects on the communities in which they are found, far beyond any immediate effects they might have on their hosts, and they are often thought of as being keystone species (e.g. D. M. Watson 2001; Crates et al. 2022). Indeed, they may sequester very subtantial amounts of photosynthate and also other nutrients produced by the host; they depend on their host for water, etc.;, amphistomaty is common here, and the stomata may be open whatever the conditions (Krasylenko et al. 2021). In at least some instances nutrients are not removed by the parasite from leaves that are about to abscise; leaf fall may be frequent and the leaves decay rapidly (see also below), and overall, there may be a considerable effect on nutrient cycling, the soil, etc., in the community (Strong & Bannister 2002; Tesitel et al. 2010c; Watson et al. 2022). Connected with this, the abundance of ground-dwelling invertebrates increases greatly in areas with mistletoes, and litter-decomposing invertebrates are conspicuous in this group; in studies where mistletoes were removed, the birds that were most affected were ground-foraging insectivores (Watson et al. 2022 and references). A variety of animals from elephants to monkeys and herbivores like eland and other bovids, eat mistletoes, sometimes notably in the dry season, and they may also more or less incidentally eat their flowers and fruits (Krasylenko et al. 2022). Flowers of mistletoes, Loranthaceae in particular, are important nectar sources attracting a variety of birds in Africa (Krasylenko et al. 2022). Since mistletoes transpire rapidly, they cool the local environment, they affect arthropod abundance, etc., and they may also provide valuable real estate for nesting and roosting birds and a variety of other animals (Watson et al. 2022). Mistletoe fruits may be valuable food resources for specialised (and other) frugivores, the seeds being dispersed by regurgitation, defaecation or bill-wiping. The fleshy fruits of mistletoes, including those of Exocarpos (Santalaceae), tend to be produced at the driest time of the year (after all, it is their hosts that are providing the means to develop this succulence) when they will presumably be maximally attractive to birds, etc., small mammals also eating the fruits (Watson 2004; Watson et al. 2011; Krasylenko et al. 2022; Crates et al. 2022).
The photosynthetic potential of mistletoes may be low relative to that of their hosts (as little as 10% in some Arceuthobium - Hawksworth & Wiens 1996), or about the same, but if low on a per unit chlorophyll basis, that may be compensated for by high chlorophyll concentrations. Transpiration rates and timing of stomatal opening may also be of interest, however, records need to be sorted out and assigned to the various clades involved (J. M. Johnson & Choinski 1993 for literature). Strong et al. (2000) suggested that the photosynthetic characteristics of the mistletoes they examined, features like a lower chlorophyll a/b ratio, lower saturating light intensity, etc., were more like those of the shade leaves of their hosts, and overall the mistletoes had a lower capacity for CO2 assimilation.
Fallen leaves of mistletoes and the excreta of organisms associated with them may enrich soil nutrients in nutrient-poor communities, mistletoe leaves facilitating the decay of recalcitrant host leaves, and overall total biomass, diversity, etc., increase (Watson 2009; Watson & Hering 2012; Ndagurwa et al. 2016, 2020). In Australia, at least, mistletoe diversity in a community is unconnected with its productivity, but host ranges are narrower in less productive (= less diverse) communities (Kavanaugh & Burns 2012). In South Africa it has been suggested that a high nitrogen content of the host, perhaps along with efficent water conductance (which will also facilitate nitrogen acquisition) may positively influence parasitism by mistletoes, and this is most frequent on species in mesic savanna communities (Dean et al. 1994). Cryptic species of mistletoe have higher concentrations of nitrogen than do non-cryptic species (Fiedler 1996; Canyon & Hill 1997). Press and Phoenix (2005) discuss interactions of Loranthaceae with their hosts, as do Monteiro et al. (2020), the latter finding that Struthanthus flexicaulis growing in Brazilian campo rupestre weakened its hosts and decreased their dominance, hence maintaining diversity overall in the community.
Hyperparasitism of one sort or another is quite common. Like other hemiparasitic groups, mistletoes quite commonly form haustoria on themselves and may parasitize other members of the order, if only on a casual basis. One plant may be found to consist of several individuals developed from seeds that germinated on the initial individual (Krasylenko et al. 2021). However, a few Loranthaceae, and a number of Santalaceae in particular, are what have been called obligate epiparasites, that is, they parasitize only other parasites. These obligate epiparasites form only a single haustorium, and that haustorium has cortical strands that allow the expansion of the parasite within the body of the host, although the overall connection between the two is notably smooth (Calvin & Wilson 2009; Wilson & Calvin 2016; Teixeira-Costa et al. 2020; Krasylenko et al. 2021).
Note that unlike many Orobanchaceae, seeds of mistletoes need no stimulant from the host to germinate (e.g. Bouwmeester et al. 2021). As with other host-parasite or host-symbiont s.l. interactions, there is considerable interest in the specificity or otherwise of mistletoe hosts. For instance, does a particular mistletoe species grow on a single/a group of related hosts, or are its hosts taxonomically diverse, and if the latter, does a wide-ranging mistletoe grow on the same diversity of hosts throughout its range (Krasylenko et al. 2022: taxa from Africa, Madagascar to the Seychelles)? Here there were some 553 genera and at least 1000 species of hosts, while there were some 26 genera and 313 species of parasites; 72 and 62 mistletoe species were found on Combretum and Ficus respectively, while about 1/3 of the host genera were associated with only a single species of mistletoe (Krasylenko et al. 2022). J. Zhao et al (2023) looked at mistletoe-host relationships in Xishuangbanna (China, Yunnan) and found that Loranthaceae were more generalists than Santalaceae, Dendrophthoe pentandra, the greatest generalist, growing on 207 host species (out of 280 total), host specificity in Santalaceae being significantly higher than in Loranthaceaae (overall: 13 spp. parasitic Loranthaceae, 270 spp. hosts, comparable figures in Santalaceae 9 and 24...).
For more on hemiparasitism in particular in Santalales, whether via roots or stems, see especially Loranthaceae and Santalaceae, and for root hemiparasitism as an apomorphy for the bulk of the order, see above and for more or some other stem parasites, see Cassthya and Cuscuta.
Seed Dispersal. The birds that disperse loranthaceous/mistletoe seeds may specialize almost entirely on fruits of this clade, being part of an association that has evolved six or more times (e.g. McKey 1975; Reid 1991; Restrepo et al. 2002; Watson & Rawsthorne 2013). Defaecation or regurgitation of the seeds, or simply leaving the sticky seed on the branch having removed the flesh of the fruit, are the three common mechanisms of dispersal (Reid 1991: Table 1). The behaviour of the disperser is often very distinctive. Flower peckers, for example, swing their bodies parallel to the branch so the seeds in their excreta land on the branch - or can be wiped off on the branch (Docters van Leeuwen 1954 in particular), and in Africa tinkerbirds (barbets) are common mistletoe eaters, and wipe the seeds sticking on their bills off onto a branch, the seeds being linked in long, dangling strings, "rosaries" (Restrepo 1987), held together by viscin (see also Reid 1983; Godschalk 1983; Polhill & Wiens 1998). In South America friar birds (Euphoniinae, near Fringillidae) commonly eat mistletoe fruits, and they also eat similar fruits from other epiphytic taxa such as Cactaceae (Rhipsalis), Araceae (e.g. Anthurium) and Bromeliaceae-Bromelioideae (Snow 1981; Restrepo 1987; Reid 1991). The seeds may pass through a bird's gut in a mere 20 minutes - some of these birds lack much in the way of a stomach at all (e.g. Reid 1991) - and when they are deposited on a branch, germination is almost immediate. Overall, about 90 species of birds from 10 families are mistletoe specialists, eating fruit of Loranthaceae and some Santalaceae (see Mathiasen et al. 2008). Watson (2020) discussed dispersal in mistletoes in general, noting that Loranthaceae tend to have larger and often red fruits and Santalaceae-Visceae, at least, smaller and often whitish fruits.
How effective mistletoe specialist birds are in dispersing seed to uninfected trees has been questioned. Thus Rawsthorne et al. (2012) found that dicaeid mistletoe specialists moved seeds of Amyema (Loranthaceae) only within forest patches that already contained the plant, since they were interested in those plants in particular. However, more generalist fruit-eaters were perhaps better at moving seeds to uninfected areas (Watson & Rawsthorne 2011; see also D. M. Watson 2022).
Plant-Animal Interactions. Santalales, especially Santalaceae and Loranthaceae, are food plants of caterpillars of some Pieridae-Pierinae-Aporiina, the so-called mistletoe butterflies (see below for mistletoes), and these include the large genus Delias, with 165-to 250+ species (all told, ca 440 species of butterflies recorded, but eating only ca 9 genera; 2/5 of all host-plant records). Loranthaceae, Opiliaceae and Santalaceae-Santaleae, -Visceae, etc., have all been recorded as hosts (Ehrlich & Raven 1964; Fiedler 1991, 1995, 1996; Congdon & Bampton 2000; Braby 2005, 2006; Braby & Nishida 2010; Burns & Watson 2013). The ancestral food plants of these pierine butterflies seems to have been mostly species of Brassicales, and their initial santalalean host plants may have been Loranthaceae (Braby 2005, 2006; Braby & Trueman 2006; Braby et al. 2006); these santalalean hosts may have been parasitic on Brassicales (Braga et al. 2021). There are no reports of pierine caterpillars on free-living Santalales, although given that there are only few such plants, this is not very surprising. The mistletoe-eating aporiine butterflies have been dated to 42 Ma (Braby et al. 2007). The origin of mistletoe feeding is dated to (57-)50, 42(-38) Ma, but Delias itself diversified only (29-)25(-23) Ma, i.e. butterfly diversification would have occurred well after that of their hosts (Braby 2006 for information); Braga et al. (2021) date Aporiina to 40-30 Ma. There is a record of the monogeneric Pieridae-Pseudopontiinae on Opiliaceae (Robinson et al., consulted vii.2015), while some pierids have switched food plants from the mistletoe to its host (Braby & Trueman 2006). Interestingly, a number of the adults of these pierines have warning colouration on the undersides of their wings, and some caterpillars also have warning colourations, but it is unclear what particular compounds the insects might pick up from their santalalean hosts that would discomfit potential predators (Braby & Trueman 2006), although there is movement of alkaloids from host to parasite in both Santalaceae and Loranthaceae (Cabezas et al. 2009). Pierids in general prefer nitrogen-rich food sources (Pellissier et al. 2012), and the foliage of mistletoes tends to be high in nitrogen, as is the foliage of Fabaceae, the original hosts of pierids. Some Aporiina have moved from Santalales back to Fabaceae (Braga et al. 2021). For more on the predilection of pierids for a high-nitrogen diet, see Brassicales.
The assocation of lycaenids with mistletoes is often forgotten, although Loranthaceae (especially), Olacaceae and Ximeniaceae are all eaten by Lycaeninae caterpillars. Lycaenid genera like Iolaus (Africa, ca 120 species) and the theclines Atlides (America: Balint 2002) and Ogyris (Australia: Pierce 1985) are associated with Loranthaceae, and larvae of the latter genus, at least, are tended by ants (see Fiedler 1995 for more records). Fiedler (1995) found that well over 10% of all host-plant records of lycaenids were on members of Santalales, most on Loranthaceae (which, proportional to its size, had most records of any angiosperm family), and most of these lycaenids were santalalean specialists. Lycaenids tend to be associated with nitrogen-fixing clades of plants since their larvae secrete carbohydrates and amino acids as food for the ants and so themselves need nitrogen-rich food (e.g. Pierce 1985); interestingly, there is some evidence that Santalaceae s.l. (including Visceae) and Loranthaceae are richer in amino acids than their host plants, moreover, they produced the non-protein amino acid hydroxyproline that their hosts did not (Greenham & Leonard 1965; see Fabaceae for lycaenids on that family, and also for non-protein amino acids). For the diversification of pierids and lycaenids, see Espeland et al. (2018 and references).
More general aspects of plant/animal interactions are not well understood, but Burns and Watson (2013) emphasized mistletoes' distinctive nature when they called them "islands in a sea of foliage"; herbivorous arthropods in particular are associated with particular species of mistletoes, and witches brooms formed by mistletoes are great habitats for many kinds of animals (see also Hawksworth & Wiens 1996; Watson et al. 2011).
[Mystropetalaceae + Loranthaceae] [Misodendraceae + Schoepfiaceae]]: acetylenic fatty acids 0, essential oils 0; cambium storied; petiole astrosclereids 0; guard cell thickenings?; epidermal cells sclerified, with druses; K minute.
Age. The age of this clade is perhaps ca 81 Ma (Vidal-Russell & Nickrent 2008a); some (74.8-)71.1(69.5) Ma is the age in B. Liu et al. (2018), 67.9 Ma in Magallón et al. (2015), and a mere 36.2/40.1 Ma in Tank et al. (2015: Table S1, S2).
[Mystropetalaceae + Loranthaceae]: ?
MYSTROPETALACEAE J. D. Hooker —— Synonymy: Dactylanthaceae Takhtajan, Hachetteaceae Doweld - Back to Santalales
Root parasites, echlorophyllous; ?chemistry; roots 0; extensive endophytic growth, vessel elements of host and parasite in contact ["simple" haustoria - D.], rhizome or tuber-like structures +, these rupture and leave a collar-like structure [= volva] at base of inflorescence; cork ?; vessel elements with simple perforation plates; stomata 0, cuticle wax crystalloids 0; leaves reduced, spiral; plant monoecious or dioecious; inflorescence terminal, axis racemose, branched or not; flowers very small, monosymmetric or not, bracts +/0, bracteoles +/0; staminate flowers: P 2-3, valvate, (basally connate); A 1-2; pollen cohesive, 3-13 pantoporate [D.]/2-4-porate [H.], pollen tube branched [?always]; pistillode 0; carpelate flowers: P 2 linear/3, connate or not; staminodes 0; G [2, 3], inferior, (initially ?2-locular), placentation apical, style single, stigma ± expanded; ovules 1-3, reduced to an embryo sac, vascular supply 0, (pseudoendothelium +), chalazal caecum ± 0 [D.]; embryo sac Polygonum-type, (chalazal caecum 0); fruits minute [≤2 mm long], drupaceous; testa 0 [D.]; endosperm cellular, ca 50-celled, chalazal haustorium single-celled, plane of first cleavage of zygote vertical[?], embryo undifferentiated, 50-85 cells [D.]; radicle hairs attach plant to host, hairs smooth, bases micropapillate [D.].
3 [list]/3. New Zealand (North Island - map in Holzapfel 2001), New Caledonia, the western Cape, South Africa.
Age. Fossil pollen of Dactylanthus has been found in deposits ca 23 Ma in New Zealand (Holzapfel 2001).
Evolution: Pollination Biology & Seed Dispersal. Short-tailed bats, Mystacina tuberculata (and introduced rats), pollinate Dactylanthus taylori in New Zealand (Ecroyd 1996; Holzapfel 2001); the bat spends a fair bit of time as a terrestrial forager. Apomixis may also occur (Hobbahn et al. 2017). There may well be environmental sex determination here with individuals switching sex - in young populations females preponderate, in older populations males come to dominate (for more information in the context of the successful translocation of this species within New Zealand, see Holzapfel et al. 2015). Indeed, all three genera of Mystropetalaceae have cohesive pollen and are probably bat-pollinaated
Genes & Genomes. Variation here - Dactylanthus is the species that has been studied - is very similar to that found in Balanophoraceae s. str., q.v..
Chemistry, Morphology, etc.. H. C. Weber (1986) noted several distinctive aspects of gross morphology and detailed anatomy of the haustoria of Mystropetalon such as runners that produced additional haustoria and graniferous tracheary cells in these haustoria that were similar to comparable structures in Santalales, however, graniferous cells occur in other root parasites including Krameriaceae and Orobanchaceae (Fineran & Ingerfeld 1982). Interestingly, the roots of the hosts of Dactylanthus form wood roses at the point of attachment like those found in Loranthaceae; these have been much harvested as curios (Holzapfel 2001).
The florally relatively unspecialized Mystropetalon has a clearly inferior ovary and the pollen is 3-5 colpate, rectangular [equatorial view]/triangular to pentagonal [polar] (Hansen 1980; see also Grímsson et al. 2017a) or (9-)12(-15)-colpate, breaking up into 3-5-angled platelets on acetolysis (Hansen 2015). Are Grímsson et al. (2017a) describing one of these platelets? Taxa like Hachettia have quite a complex fruit wall.
For additional information, see Hooker (1859), van Tieghem (1896, 1907), Kuijt (1969), Takhtajan (1988, 1997), the Parasitic Plants website (Nickrent 1998 onwards), Holzapfel (2001), Heide-Jørgensen (2008), Hansen (2015) and Nickrent (2020), all general; see also Solms-Laubach (1867) and Pellissari et al. (2022) for haustorial anatomy, Zweifel (1939), Harms (1935b), all embryogeny and Baskin and Baskin (2021) for seeds, germination, etc..
Phylogeny. Relationships are [Mystropetalon [Dactylanthus + Hachettia]] - see Su et al. (2015).
Classification. Su et al. (2015) recognised a clade that includes Mystropetalon, Dactylanthus and Hachettia as Mystropetalaceae, the other genera remained in Balanophoraceae s. str. (q.v., and whether families or not, the two groups need to be split out.
LORANTHACEAE Jussieu, nom. cons. - Back to Santalales
Inositol as storage carbohydrate; parenchyma apotracheal; flowers in triads, peduncle +, articulated, flowers sessile, subtended by recaulescent bract (+ bracteoles), medium-sized to large; K [= "calyculus"] vascularized, annular on initiation, lobes irregular, hairs on C behind anthers 0; A of different lengths [= heteranthy], adnate to base of C, anthers dorsifixed, versatile; pollen grains oblate, trilobate-triangular-convex triangular, apertures confluent [trisyncolpate]; G inferior, septate [?level], placentation basal, mamelon extended up style [?all], style long; collenchymatous zone below the embryo sacs; megaspore mother cells many [= multicellular archesporium], embryo sac growing up style (to tip); primary endosperm nucleus moving down the embryo sac, endosperm composite [derived from several ovules], aggressive, variously vertically channelled or angled, embryonic suspensor massive, long, biseriate [= biseriate proembryo], plane of first cleavage of zygote vertical, embryo chlorophyllous [?level]; n = 12, x = 6, nuclear genome [1 C] (0.298-)4.862(-79.421) pg; germination phanerocotylar.
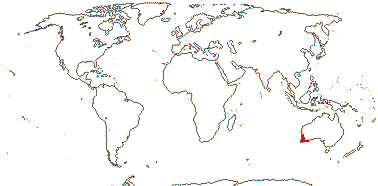
77[list]/910-950 - 6 groups below. ± Tropical.
Age. It has been suggested that the family started diversifying only 28-40 Ma (Vidal-Russell & Nickrent 2008a), however, pollen identified as Nuytsia from rocks ca 48-41 Ma in Tennessee suggest crown ages of (56.1-)50.8, 41.6(-34.2) Ma - ages in general may need to be re-evaluated (Grimsson et al. 2017b, q.v. for details). Another age is (65.6-)59.4(-52.6) Ma (B. Liu et al. 2018).
1. Nuytsieae van Tieghem - Nuytsia floribunda G. Don —— Synonymy: Nuytsiaceae van Tieghem
Tree to shrub; ellagic acid +; successive cambia +; plant with mucilage ducts; leaves spiral; plant monoecious; inflorescence racemose; C 6-8, free, yellow; A 6-8, sporangia open separately; tapetal cells 3-4-nucleate; pollen trilobate; nectary on base of style; G (-4); ovules -4/carpel; embryo sac with lateral caecum near apex; fruit dry, 3-winged; endosperm copious, ?chlorophyll, embryo ca 1/2 the length of the seed, cotyledons 3(-6), foliaceous, ?chlorophyll.
1/1. S.W. Australia. Map: from FloraBase (consulted 2006).
 [Atkinsonia [Gaiadendreae [Elytrantheae [Psittacantheae + Lorantheae]]]]: leaves opposite; fruit viscid, rubber outside vascular bundles.
[Atkinsonia [Gaiadendreae [Elytrantheae [Psittacantheae + Lorantheae]]]]: leaves opposite; fruit viscid, rubber outside vascular bundles.
Map: from Meusel et al. (1965), Jäger (1970), Barlow (1983), Polhill and Weins (1998), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010). [Photo - Flower.]
Age. The age of this node may be (57.4-)51.3(-45.7) Ma (B. Liu et al. 2018).
2. Atkinsonia ligustrina (Lindley) F. Mueller
Shrub; flowers 6-7-merous, bracts and bracteoles foliaceous; K with isolated tracheids, C yellow; pollen grains spherical; fruit drupaceous; embryo sac remaining in mamelon, with lateral caecum near apex; endosperm longitudinally furrowed, ?chlorophyllous; germination cryptocotylar.
1/1. S.E. Australia.
[Gaiadendreae [Elytrantheae [Psittacantheae + Lorantheae]]]: ?
Age. The age of this node is around (57.4-)51.3, 50.0(-44.6) Ma (B. Liu et al. 2018: tribe paraphyletic).
3. Gaiadendreae van Tieghem - Gaiadendron punctatum (Ruiz & Pavón) G. Don —— Synonymy: Gaiadendraceae Nakai
Shrub to tree (epiphytic), stoloniferous; flowers 6-7-merous; K (0), C yellow; fruit drupaceous, viscin 0; endosperm white, longitudinally furrowed; germination ?.
1/1. Montane tropical America.
[Elytrantheae [Psittacantheae + Lorantheae]]: stem parasites, shrubs, forming burl at point of attachment, epicortical runners + (0), basal, axis extension monochasial, often forming secondary burls, (shoots developing from roots), root hairs 0; (plant dioecious, flowers then small); (pedicel apex cupular); K not vascularized; C 4-6, red-yellow(-white); anthers often "long"; G [(3-)4(-5)], not septate, (style short), canal + [?distribution]; tapetal cells (uni-/bi-/multinucleate/polyploid); (G [5]); fruit baccate; endosperm chlorophyllous [?level], embryo ± plug-shaped, medium to long, (multicellular processes (also uniseriate hairs) at radicular end - ?Psittacantheae), radicle 0; chloroplast infA gene 0, rps16 g3n3 lost/pseudogenized [?level].
Age. The age of this node is estimated to be (53.2-)48.0(-42.4) Ma (B. Liu et al. 2018).
4. Elytrantheae Danser —— Synonymy: Elytranthaceae van Tieghem
(Leaves sessile, margins connate - Cyne); inflorescence operculate - C.); (endothecium 0 - Lepeostegeres); (G 4-locular, placentation axile; endosperm chlorophyllous - Lysiana).
14/164: Macrosolen (83), Decaisnina (30). South East Asia to the Antipodes, W. Pacific.
Age. The age of crown-group Elytrantheae is some (42.1-)39.3(-36.5) Ma (B. Liu et al. 2018).
[Psittacantheae + Lorantheae]: n = other than 12.
Age. The age of this node is around (52-)46.9(-41.4) Ma (B. Liu et al. 2018).
5. Psittacantheae Horaninow —— Synonymy: Psittacanthaceae Nakai
Epicortical runners also cauline, axis extension monopodial only; (stem phyllodinous); inflorescence (racemose - Tristerix); (apex of pedicel expanded); C (with basal ligule), (fenestrate, opening explosive - stylar pressure: Tristerix); A (opposite C [1 series sterile]), anthers (polysporangiate - Psittacanthus); (tapetum plasmodial, microsporogenesis successive - Cladocolea); pollen grains (spheroidal, 3-5-zonocolpate - Tupeia), (demicolpate - Dendropemon = Oryctanthus); nectary annular, on top of G/thickened style base; G oppoaite K [?level], (septate - Tripodanthus), (style solid); endosperm (0), (white - Struthanthus), embryo (undifferentiated), (cotyledons unequal); (germination cryptocotylar); n = 8 (10, 11 (Tupeia), 12).
19/335: Psittacanthus (120), Struthanthus (50), Passovia (40). New World, Baja California southwards, the Caribbean (New Zealand: Tupeia - doubtfull).
Age. Crown-group Psittacantheae are estimated to be (51.1-)44.3(-36.8) Ma (B. Liu et al. 2018: Tupeia sister to the rest).
6. Lorantheae Reichenbach
(Epicortical runners rare - Africa); (hairs dendritic/stellate); (inflorescence in involucre/surrounded by coloured bracts); C (basally connate), often fenestrate, opening explosive - staminal pressure; pollen grains (heteropolar); (heterocotyly), (tips of cotyledons fused, germination cryptocotylar); n = 9 (11).
36/400: Amyema (95), Agelanthus (60). Europe, Africa-Madagascar to China, Japan, the S.W. Pacific and the Antipodes.
Age. Crown-group Lorantheae are around (46.3-)42(-37.5) Ma (B. Liu et al. 2018).
Evolution: Divergence & Distribution. The discovery of pollen identified as Nuytsia in Tennessee, as well as other less dramatic pollen finds of Loranthaceae from elsewhere in the N. hemisphere (Grímmsson et al. 2017b; see also Manchester et al. 2015 for early pollen records) makes one wonder about the biogeography of the family (see below). Pollen grains of the pollen form-genus Aquilapollenites, bilaterally symmetrical and with four wing-like projections, are somewhat similar to those of Loranthaceae, but they are also like those of a number of other families (Farabee 1991). There is a Cretaceous circumboreal Aquilapollenites pollen province, however, the pollen has not been found associated with flowers (Friis et al. 2011 and references).
Despite this palynological connection with the northern hemisphere, it is the Australian region in particular that seems to have been important in the origin and early diversification of the family (B. Liu et al. 2018, q.v. for details): There have been at least two major radiations in West Malesia-Southeast Asia, one (?via Asia) in Africa, and one in South America. Loranthaceae are important nectar and even more important fruit sources for birds, and Liu et al. (2018) thought that their evolution might be tied up with thar of songbirds, describing a generalized coevolutionary relationship between the two. Timing is, as one might expect, tricky here. Thus meliphagids, basal oscines, are common on Old World loranths (see below), and Selvatti et al. (2015) suggest that they diverged from other oscines ca 35 Ma and speciated/radiated ca 27 Ma (Early and Late Oligocene respectively), but in Moyle et al. (2016) the crown-group age of the group is a mere 11 Ma or so. Passerida initially diversified 26-20 Ma, Zosteropidae and [Nectarinidae + Dicaeidae], the latter in particular long known for their close association with Loranthaceae, are all less than (31-)27.6, 27.1(-23.1) Ma (Selvatti et al. 2015). Surprisingly, although Loranthaceae are common and widespread in Australia today (see map above) and were, one might have thought, easily dispersed, they are unknown from Tasmania (but are found in New Zealand, New Caledonia, etc.).
Kuijt (2009b) noted that floral variation in Neotropical Loranthaceae was far greater than in Palaeotropical members. It has been suggested that sessile, axillary, 4-merous flowers were primitive in the family - genera around Phthirusa were examples (Kuijt 2011), but c.f. the character hierarchy above. Indeed, much of the discussion about inflorescence and floral morphology was carried out when there was no real understanding of the evolution of the family. It may well be that a way to understand inflorescence morphology here is to think of the main axis of the inflorescence as being indeterminate and bearing triads, pedunculate units each bearing three often sessile flowers with a recaulescent bract and two bracteoles, and these triads are variously reduced and aggregated. For discussion about the calyx/calyculus, see above.
The irregularly lobed and rim-like structure on top of the flower, sometimes called the calyculus, is thought to be of prophyllar origin in Struthanthus and Phthirusa, at least, by Wanntorp and Ronse De Craene (2009). However, evidence suggests that it is calycine in origin and it should be called a calyx (see also Eichler 1868; Kuijt 2015; Suaza-Gaviria et al. 2016), even if it is usually not vascularized (Schaeppi & Steindl 1942; Robayo et al. 2020).
The basic pollen morphology in much of the family is similar, although details of pollen surface, etc,. vary; much of the major variation is to be found in the old Psittacantheae, but resolution in this part of the tree is poor. Taxa like Tupeia and Phthirusa have spherical, 3-5-colpate pollen with an echinate surface (Grímmsson et al. 2017a: comprehensive survey). Although pollen variation correlates quite well with genera and generic groups, relating it to a phylogeny awaits a better resolution of the latter, but Grímmsson et al. (2017a) attempt this task. The root parasitic habit, as in Nuytsia, is the basal condition in the family (e.g. Vidal-Russell & Nickrent 2005); the most immediate outgroups (but not Misodendraceae) are also root parasites, as are Atkinsonia (see Menzies & McKee 1959) and Gaiadendron that appear to be near the base of the family phylogeny (see below for relationships). Teixeira-Costa et al. (2020) discuss the evolution of the various parasitic modes in Loanthaceae.
Ecology & Physiology. At the other extreme from the root-parasitic and tree-like Nuytsia is the endophytic and largely echlorophyllous Tristerix aphyllus found growing on cacti in Chile - the fused linear cotyledons up to 10 cm long form a search organ as the seedling locates its host, for instance, if the seed has landed on a spine on which it cannot grow (e.g. Mauseth 1990; Amico et al. 2007; Mauseth et al. 2011). Roots of Phrygilanthus acutifolius are described as growing down from the host into the soil, and thence to trees many metres away, which the plant then parasitizes (Benzing 1990). Teixeira-Costa et al. (2020) discuss the various ways in which loranthaceous parasites are attached to their hosts. Finally, the seeds of Tristerix corymbosus, dispersed by marsupials, can germinate on the vertical trunks as well as the branches of the host, Aristotelia chilensis is the main host, not on branchlets as is common in other Loranthaceae (Amico & Aizen 2000; Garcia et al. 2009).
It is estimated that the stem/branch parasitic habit evolved ca 28-40 Ma (Vidal-Russell & Nickrent 2006, 2008a), about when the family started diversifying (rather older estimates in Grímsson et al. 2017b, (53.2-)48(-42.4) Ma in B. Liu et al. 2018), perhaps later than in Misodendraceae (ca 75 Ma, e.g. Vidal-Russell & Nickrent 2007), although exactly when stem parasitism evolved in the Misodendraceae clade is unclear. The host-parasite junction may be much swollen, the host producing wood roses - vascular tissue in the form of variously channeled, split and branched cup-shaped structures. Morphological details of the association between stem parasite and hosts varies, and epicortical roots, which may be plesiomorphous in aerial parasites, form either sympodial (monochasial, rarely dichasial) or monopodial systems, they vary in their point of origin, etc. (e.g. Thoday 1961 and references; Polhill & Weins 1998; Calvin & Wilson 2006; C. A. Wilson & Calvin 2006b; Teixeira-Costa et al. 2020). Wilson and Calvin (2006a, b) discuss the evolution of the various kinds of host attachments; it is becoming increasingly likely that stem parasitism evolved only once in the family (c.f. Vidal-Russel & Nickrent 2008a) given the topology used here (e.g. Vidal-Russell & Nickrent 2008b; B. Liu et al. 2018; Teixeira-Costa et al. 2020). Loranthaceae are primarily xylem parasites and have very high transpiration rates, but their haustoria may sometimes tap the phloem (Barlow 1997). However, that they are xylem parasites does not mean that they cannot also take up carbon compounds, thus Marshall and Ehleringer (1990) found that Phoradendron juniperinum obtained ca 62% of its C from its host (see also Orobanchaceae, Santalaceae). Some loranths are hyperparasites, parasitizing other Loranthaceae. Host specificity is often very low (Grímsson et al. 2017b and references), thus Milner et al. (2020 and references) noted that Lysiana exocarpi had been recorded growing on 114 species in 45 genera from 21 families, although some species were preferred more than others while yet other taxa were never parasitized. On the other hand, Amyema lucasii was found only on Flindersia maculosa, while species of Muellerina prefer Callitris or Araucaria (Milner et al. 2020), although the age of that association is unclear. Desmaria mutabilis parasitizes species of Nothofagus alone in southern Chile, while Tristerix corymbosus is found on 30 or more species, but not on Nothofagus, however, it can grow as a hyperparasite on D. mutabilis... (Fontúrbel et al. 2022). See also Kuijt (2015) for further details of parasitism.
Loranthaceae have been called keystone resources, being a reliable source of both fruit and nectar for vertebrates, birds in particular, and the dense clumps of stems they form on branches are valued as nest sites by many birds (Watson 2001: eucalypt woodlands of southeast Australia; Watson & Hering 2012) (see also under Santalaceae-Visceae, Orobanchaceae, for mistletoes s.l., see above). Tristerix corymbosus is the central member in a web of ecological interactions in Patagonia, Argentina (Rodriguez-Cabal et al. 2013). The flowers of this mistletoe provide the only nectar during winter for the hummingbird Sephanoides sephanoides - there is an obligate pollination mutualism here - which at other times of the year is an important pollinator of other plants in the area. Similarly, the marsupial Dromiciops gliroides is the only known disperser of the seeds of T. corymbosus, and these often end up on the host of Tristerix, Aristotelia chilensis, and Dromiciops also disperses the seeds of a number of other genera - and this whole complex system is being broken down by introduced cows and other ungulates (Rodriguez-Cabal et al. 2013).
In Australia, the shapes of loranth leaves and of the eucalyptus host on which they grow are often similar (Barlow & Wiens 1977), although Loranthaceae tend not to be very specific as regards their hosts (see above). Explanations for this phenomenon, at most uncommon elsewhere, vary. Canyon and Hill (1997: p. 395) examined this phenomenon in detail, concluding "[Our] results contradict, in some crucial aspect, all of the mimicry hypotheses currently on offer". Indeed, other work suggests that mimicry is not involved (Blick et al. 2012), but M. E. Cook et al. (2020) thought that it did occur... For other possible cases of host—parasite mimicry, see Alseuosmiaceae and Lardizabalaceae.
Leaf flush in Struthanthus may be more pronounced if its host is deciduous (Y.-B. Zhang et al. 2023).
Pollination Biology & Seed Dispersal. Loranthaceae are a major source of both nectar and fruit for birds throughout the tropics. There are about 200 species of bird-pollinated Loranthaceae in Africa (Polhill & Wiens 1998), about 70 species in Australia (Barlow 1984), 36 in China (Qiu & Gilbert 2003), 165 in Malesia (Barlow 1998), and 125 in the New World. Most species with large flowers are pollinated by birds, those with small flowers - often dioecious - are pollinated by bees, etc. (Suaza-Gaviria et al. 2016). 44 species in two genera of Nectariniidae-Dicaeidae endemic to the Indo-Australian area are involved in both pollination and seed dispersal of the family (e.g. Docters van Leeuwen 1954; Reid 1983). In some Loranthaceae from both the Old and New World the flower opens only when the bud is squeezed or pecked at by the birds (hence the common name of Dicaeidae, flower peckers), opening being by rapid elongation of the initial slits (fenestrae) apparent in the flower buds. This opening can be explosive, the bird becoming showered with pollen from the anthers which have already opened in bud. Some Loranthaceae are generalists, while in others the shape of the bird's bill and that of the corolla tube match, and then pollination is more restricted (e.g. Feehan 1985). Other very common pollinators in the family are sunbirds (Nectariniidae-Nectariniini), closely related to Dicaeidae and also Old World, and in Malesia they pollinate species whose flowers do not open explosively (Corlett 2004). Sunbirds are the main pollinators of most African Loranthaceae, but explosive pollination does occur there; Kirkup (1998) provides a detailed study of the variety of floral morphologies involved. In tropical America and temperate South America hummingbirds are the major pollinators (Kuijt 2015). There is also explosive opening of the flowers in the New World Tristerix (see also Abrahamczyk et al. 2017, pollination of Chilean species), and there the initial slits in the corolla tube are caused by pressure exserted by the style, while in Old World taxa pressure from the stamens causes the slits to appear (literature in González & Pabón-Mora 2017c). Aetanthus and Tristerix are part of the pollination guild in which the very long-billed hummingbird Ensifera ensifera is involved (Soteras et al. 2018). During the winter in Patagonia T. corymbosus is the only source of nectar for Sephanoides sephanoides. For bird-pollination of New Zealand Loranthaceae, see Ladley et al. (1997). Vidal-Russell and Nickrent (2008b) discussed the evolution of bird-pollinated flowers in the family, which, they thought, had occurred several times. Interestingly, the basal root-parasitic Loranthaceae have yellow, insect-pollinated flowers (Watson 2020). Pseudanthia are known from Tolypanthua ((Baczynski & Claßen-Bockhoff 2023).
For seed dispersal, see also above. The birds that disperse loranthaceous/mistletoe seeds may specialize almost entirely on fruits of this clade, being part of an association that has evolved six or eight or so times (e.g. McKey 1975; Reid 1991; Restrepo et al. 2002; Watson & Rawsthorne 2013), perhaps within the last 25-20 Ma (references in Amico & Aizen 2000). Defaecation or regurgitation of the seeds, or simply leaving the sticky seed on the branch having removed the flesh of the fruit, are the three common mechanisms of dispersal (Reid 1991: Table 1). The behaviour of the disperser is often very distinctive. Flower peckers, for example, swing their bodies parallel to the branch so the seeds in their excreta land on the branch - or can be wiped off on the branch (Docters van Leeuwen 1954 in particular), and in Africa tinkerbirds (barbets) are common mistletoe eaters, and wipe the seeds sticking on their bills off onto a branch, the seeds being linked in long, dangling strings, "rosaries" (Restrepo 1987), held together by viscin (see also Reid 1983; Godschalk 1983; Polhill & Wiens 1998). In South America friar birds (Euphoniinae, near Fringillidae) commonly eat mistletoe fruits, and they also eat similar fruits from other epiphytic taxa such as Cactaceae (Rhipsalis), Araceae (e.g. Anthurium) and Bromeliaceae-Bromelioideae (Snow 1981; Restrepo 1987; Reid 1991). The seeds may pass through a bird's gut in a mere 20 minutes - some of these birds lack much in the way of a stomach at all (e.g. Reid 1991) - and when they are deposited on a branch, germination is almost immediate. Overall, about 90 species of birds from 10 families are mistletoe specialists, eating fruit of Loranthaceae and some Santalaceae (see Mathiasen et al. 2008). Watson (2020) discussed dispersal in mistletoes in general, noting that Loranthaceae tend to have larger fruits that are often red and Santalaceae-Visceae, at least, smaller and often whitish fruits. The fruits of mistletoes are high in lipids and proteins, and the birds feed them directly to their young (c.f. hummingbirds), and of course the fruits are often available the year round (Watson & Rawsthorne 2013 - integrate).
However, how effective mistletoe specialists are in dispersing seed has been questioned, especially to new areas with uninfected trees. Thus Rawsthorne et al. (2012; see also Watson & Rawsthorne 2011, 2013) found that dicaeid mistletoe specialists like Dicaea hirundinaceum moved seeds of Amyema preissii (Loranthaceae) only within patches that already contained the plant. It was more generalist fruit-eaters that were better at moving seeds to trees in uninfected areas.
Interestingly, the fruits of both Tristerix corymbosus (just one large seed) and Desmaria mutabilis (Loranthaceae) in Chile are green and they are an important source of food for the marsupial Dromiciops bosinovici, a nocturnal feeder (Amico & Aizen 2000; Fontúrbel et al. 2022).
Plant-Animal Interactions. Loranthaceae are the major hosts for caterpillars of pierid and lycaenid butterflies (see introduction to Santalales for literature), some other hemiparasitic Santalales also being involved. Lycaenidae-Iolaini caterpillar preferences show fair agreement with the classification of Polhill and Wiens (1998), in particular, most Iolaini are found either on tapinanthoid or taxilloid genera, the two main African groups of the family (Congdon & Bampton 2000). Caterpillars of the pierid Delias occur on Malesian Loranthaceae (Docters van Leeuwen 1954) and of Mylothris on African Loranthaceae (Braby 2005).
Elephants like to eat Loranthus, and will knock over Acacia (= Senegalia) trees to get at the plant (White 1983); as noted above, the parasites may be quite rich in nitrogen (see also goats, elephants, primates and Santalaceae-Visceae).
Genes & Genomes. Mitochondrial genes from a presumably root-parasitic member of Loranthaceae seem to have been acquired by the fern Botrychium virginianum (Ophioglossaceae), perhaps via a common mycorrhizal associate (Davis et al. 2005b). However, in general mycorrhizae are not very common on Santalales and if the gene transfer took place in Asia, as Davis et al. (2005b) suggested (this is where the fern may have originated), the absence of extant root-parasitic Loranthaceae from that area is noteworthy.
Economic Importance. Mathiasen et al. (2008) provide a list of Loranthaceae that harm crops - citrus and cocoa are particularly susceptible.
Chemistry, Morphology, etc.. González and Pabón-Mora (2017c) describe the flower of Tristerix as if its orientation were inverted, i.e. the odd petal is adaxial. The apex of the "pedicel" - but see Divergenca & Distribution above for inflorescence morphology - is quite often swollen and forms a cupular structure that may be accrescent in fruit (Suaza-Gaviria et al. 2016). Polysymmetric 6-merous flowers seem to be plesiomorphous in the family (Barlow 1983; C. A. Wilson & Calvin 2006a; Suaza-Gaviria et al. 2016), but 7-(or 8-)merous flowers occur in Atkinsonia. There is some variation in nectary morphology, and formation of the corolla tube may be postgenital, via interdigitating hairs on the edges of the petals (Robayo et al. 2020). Stamen dimorphism - even although there are only as many stamens and petals - is supposed to be almost exclusively a New World phenomenon (Kuijt 2010), but the androecium of e.g. Australian taxa like Nuytsia and Atkinsonia are described as being in two series. Anther loculi dehisce independently in Peraxilla (Prakash 1960).
For some discussion about ovules and embryology, see above. The imbalance of embryological work carried out on Old Word and New World Loranthaceae is extreme. As an example of possible surprises in the latter, Venturelli (1981) found that Struthanthus produced only a single embryo sac and so compound endosperm could not form there, apparently unique in the family (it was formed in Tripodanthus - Venturelli 1983). Cronquist (1981) and others describe the gynoecium as being 3-4-carpelate with 7-12 ovules; see Robayo et al. (2020) for those taxa that have septae. For literature about the mamelon, a structure in the centre of the ovary in aseptate taxa, over whether or not it is at least part placental or ovular in nature (the former is surely likely), see e.g. Narayana (1959) and Suaza-Gaviria et al. (2016). The mamelon may be vascularized or not (Narayana 1959: as also in Dendrophthora - Santalaceae-Visceae). The embryo sac in Moquiniella is some 48 mm long, the longest in the angiosperms; it grows up the style and then may grow back downwards a little after reaching the stigma (this is sometimes called an embryo sac haustorium - see Mikesell 1990), and other members of the family have embryo sacs nearly as long (e.g. Johri & Bhatnagar 1972; Cocucci 1990; Johri et al. 1992). In general, after fertilization the embryo is "planted" back down at the base of the mamelon by the development of a long, biseriate suspensor. Polar and primary endosperm nuclei can also be peripatetic. Thus Venturelli (1981) described how one polar nucleus migrated up the embryo sac in Struthanthus, and after double fertilisation there was apparently an uniseriate strand of endosperm cells in the style, endosperm developing back down in the ovary - however, Johri et al. (1992) in their summary of the literature suggested that the primary endosperm nucleus migrated to the base of the embryo sac, and this migration is indeed mentioned in a number of accounts. The embryo sac quite often has a caecum of some sort, e.g., there is a basal caecum in Lysiana and Lepeostegeres, the antipodal cells being lateral, and the endosperm develops in the caecum (Narayana 1958; Dixit 1959a; Prakash 1960). There is an hypostase, a disc of sclerenchymatous cells at the base of the nucellus, and this seems to limit the extent of the downward growth of the embryo sac and associated structures (see Robayo et al. 2020). Both Cronquist (1981) and Takhtajan (1997) - and many primary sources - describe the endosperm as being starchy (e.g. Dixit 1959a, b), but it is not so scored in Malécot (2002); the embryo sac may also be full of starch grains. The endosperm is aggressive, and adjacent tissues become obliterated. However, the vascular bundles are resistant, the result being a mature endosperm that is almost a variant of ruminate endosperm - it is longitudinally furrowed, the furrows marking the position of the vascular bundles. The endosperm may be merely angled in transverse section, as in Amyema (Dixit 1959a). Kuijt (1982) was perplexed by the cotyledons of Psittacanthus ramiflorus which, he thought, showed infraspecific variation - there were either two flat cotyledons, or 7-10 prismatic cotyledons. However, González and Pabón-Mora (2017a) suggested that the persistent reports of polycotyledony in Psittacanthus were incorrect, the "extra" cotyledons being lobes of green endosperm formed in the way just described, and although Kuijt (2017) thought that González and Pabón-Mora had misinterpreted morphology in the context of misidentified seedlings, González and Pabón-Mora (2017d) defended their interpretation - this all needs to be cleared up (see also Ornelas et al. 2024). In many Old World Loranthaceae the cotyledons are connate, but not basally; the plumule emerges through the basal slit (Kuijt 1969). The embryos of Helicanthus, Lysiana, and some other genera have multicellular processes and uniseriate hairs at the radicular end (e.g. Johri et al. 1958; Narayana 1958), and a true radicle may not be present (Prakash 1960).
For much information about all aspects of the family, see Johri and Bhatnagar (1972) and Kuijt (2015), for Nuytsia, see Hopper (2010), for Psittacanthus, see Kuijt (2009a), for anatomy of wood, see Sibinelli and Ceccantini (2022), of haustoria, see H. C. Weber (1984), and leaf, see Kuijt and Lye (2005: terminal tracheids of veinlets), for growth habits, see Benzing (1990), for floral anatomy, see Robayo et al. (2020) and Lamilla et al. (2020) and references, for floral morphology and embryology, see Schaeppi and Steindl (1942), Robles et al. (2016), González and Pabón Mora (2019) and Lamilla et al. (2020: Tristerix), for pollen, see also Feuer and Kuijt (1985 and references), and for embryology, etc., see Treub (1882: Loranthus), Singh (1951: Dendrophthoe), Maheshwari and Singh (1952), Narayana (1959: Nuytsia), Dixit (1959a, b, 1962), Prakash (1962: Atkinsonia, 1963), Raj (1970), Bhatnagar and Johri (1983) and Subrahmanyam et al. (2015).
Kuijt (2015) noted that the seedlings of genera from the Old Word were not much known, nor the embryology of those of the New World.
Phylogeny. Relationships within Loranthaceae are slowly being clarified. There is good support for the position of Nuytsia as sister to the rest of the family (Vidal-Russell & Nickrent 2005; Su et al. 2015; B. Liu et al. 2018; Nickrent et al. 2019). Immediately beyond this, things are a bit murky. The root parasitic Atkinsonia (S.E. Australia) and Gaiadendron (Central and South America) are also near the base of the phylogeny (Liu et al. 2018: ML bootstrap support low). However, there was rather weak support for the stem parasite Notanthera being sister to all Loranthaceae except Nuytsia (C. A. Wilson & Calvin 2006a, b), both Vidal-Russell and Nickrent (2008b) and Liu et al. (2018) placed this in Psittacantheae. Su et al. (2015) also recovered Nuytsia and Atkinsonia as succesively sister taxa to the rest of the family (support for the latter position weak), and Gaiadendron again seemed to be in this area. Grímsson et al. (2017a, b) thought that basal relationships in the family were unclear, Grímsson et al. (2017a) noting that the positions of the root-parasitic Gaiadendron and Atkinsonia were uncertain, and they thought that Tupeia might be sister to the rest of the family - but see Liu et al. (2018). Relationships remained unclear in Nickrent et al. (2019), althoough the same list of suspects was involved.
Relationships in Psittacantheae (?basal, ?paraphyletic) were unclear, but Elytrantheae included two well supported clades and the bulk of the family was included in a well supported Lorantheae within which there was a moderate amount of structure (Grímsson et al. 2017a). Indeed, although support for relationships along the spine ranged from strong (PP) to rather poor to strong (ML bootstrap) in B. Liu et al. (2018), support values for both monophyly and basal relationships in Elytrantheae and Psittacantheae and for basal relationships in Lorantheae were low, and the monophyly of a number of genera remains to be established.
Classification. For a suprageneric classification of the whole family, see Nickrent et al. (2010); for some generic limits, see Kuijt (2011). It will be clear from the above discussion that support for elements of the classification above, basically that of Nickrent et al. (2010), are weak.
Previous Relationships. Loranthaceae and Viscaceae (see Santalaceae below, as Visceae) have often been considered to be close, even being put in a single family, but there are numerous features separating the two; Kuijt (1969), Raj (1970) and Polhill and Wiens (1998) provide useful tables of differences. Thus the viscous covering of the seeds of Loranthaceae is mesocarpial in origin, being outside the vascular bundles and, the fruits of Loranthaceae contain rubber (and are sometimes used as bird lime). Although some Loranthaceae such as Phthirusa have very small flowers and congested inflorescences, they are easily separated from Visceae which also have these features; plants of the latter are often lighter green in color, they lack roots running over the surface of the host, their flowers are often three merous, and they have green endosperm, etc..
[Misodendraceae + Schoepfiaceae]: style ?hollow.
Age. The age of this node is ca 75 Ma (Vidal-Russell & Nickrent 2006, 2007) or ca 58 Ma (Magallón et al. 2015).
Phylogeny. Kuijt (1968) rejected the ides of a close relationship between Misodendraceae and Schopfiaceae (Quinchamalium) because of differences he saw between their female gametophytes and endosperm haustoria.
MISODENDRACEAE J. Agardh, nom. cons. - Misodendrum de Candolle - Back to Santalales

Stem parasites; successive cambia + [cambia develop centripetally], cuticular epithelium 0; sieve tube plastids lacking starch and protein inclusions; wood rayless [?all]; sieve tube plastids lacking both starch and protein inclusions; bundle fibres +; ?stomatal orientation; leaf mesophyll undifferentiated; hairs 0/unicellular; stem apex aborting; plant dioecious; staminate flowers: pedicel +; K 0, C 0; A 2-3, monothecal, dehiscing by apical slit; pollen grains spheroidal, 4-19-pantoporate, pores operculate, surface spinuliferous; pistillode inconspicuous; carpelate flowers: sessile; K 0, ?C 3, minute; staminodes alternate with C; style ± 0, stigma strongly 3-lobed; ovules straight; fruit dry, achenial, attached to 3 much accrescent long-plumose staminodes growing from slits in the ovary; endosperm chlorophyllous, with a single nucleate haustorium that branches in the receptacle, "hypocotylar" zone very broad, cotyledons ± connate, radicle replaced by sticky sheath; n = 6, 8, x = ?; germination ± cryptocotylar.
1 [list]/8. Cool temperate South America. Map: from Heywood (1978). Photos - Misodendrum Flower, Misodendrum Habit.
Evolution: Divergence & Distribution. Pollen of Misodendraceae has been found in Patagonia in deposits ca 45 Ma (Del Carmen Zamaloa & Fernández 2016).
Ecology & Physiology. Stem parasitism may have evolved here ca 75 Ma (e.g. Vidal-Russell & Nickrent 2007) well before that in Loranthaceae or other mistletoes, its origin in Loranthaceae being dated to some ca 40 Ma (Vidal-Russell & Nickrent 2006, 2007) or - less of a difference - (53.2-)48(-42.4) Ma (B. Liu et al. 2018). However, exactly when stem parasitism evolved after the separation of this clade is unclear. Somewhat unusually, Misodendron neither hosts other parasites nor is an epiparasite - see also Arceuthobium Krasylenko et al. 2021). For mistletoes in general, see above.
Seed Dispersal. Seeds are dispersed by wind, unlike those of other mistletoes, or they stick to passing animals (Reid 1991 and references).
Chemistry, Morphology, etc.. The inflorescence may look like a (compound) raceme or spike, but there are clearly cymose units in some species (Suaza Gaviria et al. 2017). The most prominent stucture in the staminate flower is the pedicel. According to Takhtajan (1997) the pollen is colpate. There are reports that the chalazal endosperm haustorium is multicellular ().
For general information, see Kuijt (1969; 2015: in the latter the ovary is described as being superior), for details of wood anatomy, which is rather distinctive, see Carlquist (1985c), for pollen, see Del Carmen Zamaloa and Fernández (2016 and references), and for floral morphology, embryology, etc., see Skottsberg (1914).
Phylogeny. For a phylogeny of Misodendrum, see Vidal-Russell and Nickrent (2007). Misodendron quadriflorum is sister to the rest of the genus, thus making subgenus Angelopogon paraphyletic - c.f. earlier classifications, including that in Zavaro et al. (1997: morphological cladistic analysis).
SCHOEPFIACEAE Blume —— Synonymy: Arjonaceae van Tieghem - Back to Santalales
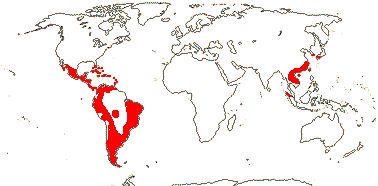
(Perennial herbs); aliform confluent parenchyma +; (nodes 1:3); epidermal cells not lignified, (stomata anomo-/cyclocytic); hairs unicellular/0; bract and bracteoles usuaully immediately below and surrounding G, connate; flowers heterostylous [distylous], medium-sized; (K +, shallowly lobed]), C tubular, ± connate, hairs on C abaxial to A (Quinchamalium - Q.); pollen grains tetrahedral, heteropolar, apertures ± confluent, (zonasulculate)/triporate [Q.], ektexine smooth; G (semi-)inferior, style?, stigma lobed to capitate; ovules ategmic; embryo sac U- or J- [Q.] or open heart-shaped [Schoepfia], chalazal haustoria branched [Q.], (synergid haustoria +, very slender, growing up style - Q.); archesporium multicellular [Q.], chalazal cells in chamber, chalazal haustorial processes branched [Q.]; embryo short to long, first division vertical/oblique; chalazal endosperm haustoria 4, unicellular, slender, endosperm with starch, oil; embryo long [Q..]; n = 12, 14, x = ?
3 [list]/55: Quinchamalium (25). Central and South America, a few species in tropical South East Asia-West Malesia. Map: from Sleumer (1984); South East Asian mainland and South America only approximate distributions. [Quinchamalium flower.]
Age. Fossil Schoepfia is reported from Eocene deposits ca 50 Ma in the Okanogan Highlands in W. North America (Princeton, Republic: see Wehr & Hopkins 1994).
Evolution: Divergence & Distribution. To say that Quinchamalium has a remarkable embryo sac/embryogenesis is an understatement, even by santalalean standards (see Johri & Agarwal 1965 for details).
Pollination Biology. Heterostyly is reported from Schoepfia schreberi and Quinchamalium chilense (Cohen 2019), Simón--Porcar et al. (2024) noting that it was found in all genera there.
Chemistry, Morphology, etc.. Sleumer (1984) noted that the wood had aliform-confluent parenchyma, unlike other "Olacaceae".
The synergid haustoria of Quinchamalium grow about a third of the distance up the style (Johri & Agarwal 1965). There are prominent bracteoles immediately below the flowers which look not unlike those of Loranthaceae. On the other hand, Johri and Agarwal (1965) described the calyx of Quinchamalium as being made up of four sepals, each with a vascular bundle; the adaxial member was largest - not onbiously bract + bracteole. The calyx and pollen of Schoepfia are also similar to those of Loranthaceae. However, pollen aperture development in Schoepfia follows Garside's Rule, there being three pores at four points in the tetrad (see Blackmore & Barnes 1995) - other genera? Agarwal (1962) and Johri and Agarwal (1965) described the integument as being "massive", but there is neither micropyle nor obvious integument.
For additional information, see Dawson (1947 and Kuijt (2015), both general, also Swamy (1949c: vegetative anatomy and pollen), F. H. Smith and Smith (1943: floral morphology), Jarzen (1977: pollen of Arjona), Grímsson et al. (2017a: pollen, extensive variation), and Watanabe (1943: Schoepfia), Agarwal (1961b: Quinchamalium), Johri and Agarwal (1965 and references: family) and Bhatnagar and Sabharwal (1969: Jodina), all embryology.
Schoepfiaceae are very poorly known.
Phylogeny. That Schoepfia is rather different from "Olacaceae" had often been remarked (e.g. Metcalfe & Chalk 1950; Sleumer 1984). Arjona, ex Santalaceae, was found to be sister to Schoepfia (Malécot 2002), and Quinchamalium, another ex Santalaceae, is also to be included here. Support for the relationships [Schoepfia [Arjona + Quinchamalium]] is strong (Vidal-Russell & Nickrent 2006; Der & Nickrent 2008); Smith and Smith (1943) had noted relationships between Quinchamalium and Schoepfia.
[Opiliaceae + Santalaceae]: single perianth whorl [= C]; chloroplast infA gene 0.
Age. Bell et al. (2010) dated this node to (106-)89, 82(-64) Ma, Wikström et al. (2001) suggested an age of (85-)80, 69(-64) Ma, and Tank et al. (2015: Table S2) an age of a mere 45/44 Ma, by far the youngest.
Chemistry, Morphology, etc.. The single perianth whorl - very common in this clade - is probably equivalent to the corolla of other members of the order, where the calyx is often small; Hiepko (1984) called this single whorl the perianth in Opiliaceae only because there was no obvious calyx.
OPILIACEAE Valeton, nom. cons. —— Synonymy: Anthobolaceae Dumortier, Cansjeraceae J. Agardh - Back to Santalales
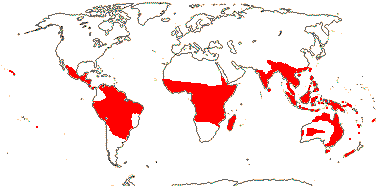
Trees or shrubs (lianes); tanniniferous?; wood often fluorescing; nodes (1:1), 1:3, 1:5; silicification of mesophyll cells 0; cystoliths + (0); stem stomata transversely oriented [Anthoboleae]; leaves two-ranked to spiral; inflorescences axillary, (racemose/spicate/catkin-like, with relatively large bracts), (plant dioecious); bracteoles 0; flowers small, (3-)4-6(-8)-merous; hypanthium + or 0; C free (± connate), (0 in carpelate flowers); A = C, adnate to C or not; tapetal cells 4-nucleate; pollen usu. colporate, microechinate, spheroidal, triangular; (prominent nectaries alternating with A); G [2-5], ovary on elongated receptacle, style (0), hollow, stigma ± capitate; ovule 1(2), pendant, erect, basal [Agonandra], 1≤, on mamelon [Anthobolus], with micropyle [Cansjera]; micropylar caecum almost breaks through the "integument"; (pedicel swollen, coloured - Anthobolus); (endosperm haustorium with dendritic branches developing), (additional unicellular secondary endosperm haustoria with dendritic branches - Cansjera), endosperm also oily, embryo narrow, long, (rather short), radicle very short, cotyledons (2-)3-4; germination cryptocotylar; n = 10, x = 10 (?5).
12 [list]/36. Pantropical (map: from Stauffer 1959; Hiepko 1984, 2000, M. Gustafsson pers. comm. ii.2010 - Africa; FloraBase x.2012; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010). [Photo - Flower]
Evolution: Divergence & Distribution. Le et al. (2019) examined the distributions of a number of morphological characters on their tree, Strombosia, rather distant, was the outgroup.
Chemistry, Morphology, etc.. Metcalfe and Chalk (1950) describe a branching system of lignified cells connecting veins in Olacaceae s. str., i.e. not including Anthobolus. Agonandra and Anthobolus have amphistomatic leaves and green twigs.
Cansjera has a basal ovule (Swamy 1961), and this can also be interpreted as an ovule on a much reduced mamelon. The basal part of the gynoecium elongates greatly before anthesis, leaving the ovary + stigma-style perched on the end of quite a long podium (Swamy 1961). Stauffer (1959) suggested that there might be viscin in the fruits of some Anthoboleae.
For embryology, see also Swamy and Dayanand Rao (1956), Swamy (1961) and Ram (1970), and for general information, see Stauffer (1959), Hiepko (1984) and Kuijt (2015).
Phylogeny. The Australian Anthobolus, ex Santalaceae, is to be included here, relationships being [Lepionurus (support strong) [Anthobolus + The Rest (support weak)]] (Der & Nickrent 2005, esp. 2008, also Z. Zhou et al. 2019); it, like other Opiliaceae, has a superior ovary. Le et al. (2019) in a study that included all but one genus of the family found that Agonandra, probably also with Gjellerupia, was sister to the rest of the family, and in one clade Anthobolus was sister to Champereia etc., but in neither case was support strong. Relationships in Nickrent et al. (2019) were largely pectinate but poorly supported; Agonandra waas sister to the rest of the family.
Classification. Le et al. (2018) propose a tribal classification for the family - 3 tribes, 12 genera, 36 species - but given the poor support in the basal nodes I do not follow it.
Previous Relationships. Stauffer (1959), who monographed Anthoboleae, considered that they were closer to Santalaceae than to Opiliaceae; Anthoboleae, wihout Anthobolus, are indeed close to Santalum and relatives (Der & Nickrent 2008: support strong).
SANTALACEAE R. Brown, nom. cons. - Back to Santalales
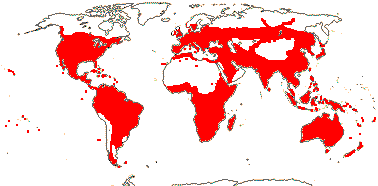
Ellagic acid 0; axial parenchyma strands 0; cuticular epithelium common; (cuticle waxes as rodlets); stomata often transeversely oriented, guard cell thickenings unknown; epidermal cells sclerified, with druses; flowers small, (3-)4-5(-8)-merous; hypanthium + or 0; K 0, hairs on C behind A, unicellular, base swollen (0); large nectary glands often +, alternating with stamens; pollen various; G [2-5], inferior, odd member abaxial, (carpellary vascular supply recurrent), stigma often capitate or lobed; ovules straight (anatropous), or not distinguishable, micropylar end ± protruding; fruit drupaceous [mesocarp stony], also baccate, (outer part exfoliating); endosperm (helobial), starchy or not, embryo short to long; x = 7 (?6. ?5), nuclear genome [1 C] (0.104-)2.128(-43.57) pg.
44 [list, tribal assignments]/990 - seven groups below. World-wide, esp. tropics. Map: see Meusel et al. (1965), Hawksworth and Wiens (1972), Fl. Austral. vol. 8 (1984), Jalas and Suominen (1976), Polhill and Weins (1998) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
[Cervantesia group [Thesieae + Comandra group]]: chalazal haustorium uninucleate.
1. Cervantesia group —— Synonymy: Cervantesiaceae Nickrent & Der
(Axillary thorns +/?spine - Scleropyryum; (gallic acid +); (nodes 1:3); (lamina rhombic, with spines - Jodina); (plant dioecious); (inflorescence racemose - S.); (A bifid - S.); pollen 2-celled; tricolpate (-colporate, surface striate - Buckleya), (heteropolar); tapetal cells 2-4 nucleate, nuclei fused; nectary lobes between C/0; G [3], superior to inferior, placental column twisted/straight, style (short/0), stigma lobed, papillate; ovules anatropous, epitropous, micropyle long, integument 4-7 cells across; embryo sac bisporic, 8-nucleate [Allium-type]; chalazal haustorium long [S.]; fruit surrounded by C, longitudinally ridged, C dehiscent/smooth, mesocarp sclereidal; first division of primary endosperm cell unequal, micropylar cell smaller, chalazal cell with copiously-branched protrusion, nucleus huge; embryo mid-sized; n = ?
10/21. Tropical, warm temperate, esp. America. [Photos - Acanthosyris fruit.]
[Thesieae + Comandra group]: placental column long, twisted; ovules +, unitegmic; embryo sac confined to ovule; endosperm cellular.
2. Thesieae Meisner —— Synonymy: Thesiaceae Vest
Plant annual herbs to (scandent) shrubs; ?santalbic acid +, (bufadienolides +); (axillary thorns +); (lamina terete); (plant dioecious); flowers 4-5-merous; (K +, strongly lobed); (C connate); pollen grains (3-colporate), (heteropolar); stalk of "placenta" twisted; embryo sac not extending beyond micropylar region, chalazal caecum growing down mamelon base; fruit drupaceous [stony layer = mesocarp], testa 0; endosperm composite/two chambers, massive, starchy/aleurone, chalazal haustorium (-several-celled), branched deep in receptacle, zygote takes a long time to divide [T.], first division longitudinal), embryo (suspensor 0), length medium-long; n = 6-9(-12).
5/345: Thesium (345). More or less world-wide, not Arctic, esp. southern Africa.
3. Comandra group —— Synonymy: Comandraceae Nickrent & Der
Plant herbaceous; (hypanthium +); endothecial thickenings poorly developed; (pollen grains (3-colporoidate); nectary lobes between C; placental column long, twisted, stigma punctate to subcapitate; ovules anatropous, unitegmic; (epicarp dry); secondary endosperm haustoria +, zygote with first division vertical; germination cryptocotylar; n = 13 (14).
2/2. North America, Europe and the Mediterranean, ± temperate.
[Nanodea group [Santaleae [Amphorogyneae + Visceae]]]: ?
4. Nanodea group —— Synonymy: Nanodeaceae Nickrent & Der
?Santalbic acid; (flowers 4 merous); (K +); pollen heteropolar, etc. [Mida]; tapetal cells mulyinucleate; nectary lobes between C; G (semi-)inferior; micropylar endosperm +, cells with dendritic haustoria, 1-celled secondary chalazal endosperm cells parallel to caecum; embryo small, suspensor massive, multicellular; n = ?
2/2. New Zealand, south South America.
[Santaleae [Amphorogyneae + Visceae]]: (stem parasites +); (ovule reduced to embryo sac).
Age. The age of this node is some (71-)67, 65(-61) or (57-)53(-49) Ma (Wikström et al. 2001) or just over 135 Ma (Maul et al. 2018).
5. Santaleae Dumortier —— Synonymy: Canopodaceae C. Presl, Eremolepidaceae Nakai, Exocarpaceae J. Agardh, Lepidocerataceae Nakai, Osyridaceae Rafinesque
(Stem parasites, epicortical roots +/0); (nodes 1:3); cuticular epithelium + [?all]; (phylloclades +); (leaves spiral), (scale-like); flowers (unisexual), (sessile); flowers 3-6-merous, (monosymmetric); (K +), hairs at the base of A/0; pollen grains (3-porate/colporate), (surface spinuliferous), (ellipsoid, colpi narrow, but ± joining equatorially); nectary massive, ± alternating with A; G ± inferior, mamelon beaked or not; (ovary 1-locular, embryo sacs 2, basal), (ovules oriented towards style - Osyris), style hollow; parietal tissue 2 cells across/connate integuments; embryo sac (not extending beyond ovule), (bisporic, 8-nucleate [Allium type]), chalazal caecum growing down mamelon base; (pedicel swollen, fleshy in fruit); (seed with a complete viscous covering), testa 0; endosperm (helobial), (0), (composite [derived from several ovules] - Santalum), (chalazal haustorium multicellular - Exocarpus), (chlorophyllous), embryo (large), (undifferentiated - Lepidoceras), suspensor long/0, (primary [radicular] haustorium +); n = 10, 11, 13, 15, chromosomes "very small" [Antidaphne].
11/51: Exocarpos (26). Widely scattered, not N. temperate.
[Amphorogyneae + Visceae]: (plants hyperparasitic (obligate epiparasites)); (C basally connate); stamens with short/0 filaments; pollen grains 3-colporate; G inferior.
Age. Stem Visceae, i.e. the age of this node, are some 72 Ma old (Vidal-Russell & Nickrent 2008), while (156.3-)124.7, 102.1(-68.5) Ma is estimated by Maul et al. (2018).
6. Amphorogyneae Stearn —— Synonymy: Amphorogynaceae Nickrent & Der
(Stem parasites); pyrrolizine alkaloids +; (leaves opposite), lamina with ± palmate (pinnate) venation, (linear), (much reduced); (plant dioecious); flowers 4-6-merous; anther loculi above one another in pairs, all four sporangia form common chamber [Leptomeria]; tapetal cells binculeate; (nectary lobed); G [5], mamelon not beaked, style short; endosperm not starchy; (embryo very small); n = ?
9/68: Dendromyza (21), Leptomeria (17), Phacellaria (8). Southeast Asia, Malesia, Australia and New Caledonia.
7. Visceae Horaninow —— Synonymy: Arceuthobiaceae Nakai, Bifariaceae Nakai, Dendrophthoraceae van Tieghem, Ginalloaceae van Tieghem, Phoradendraceae H. Karsten, Viscaceae Batsch
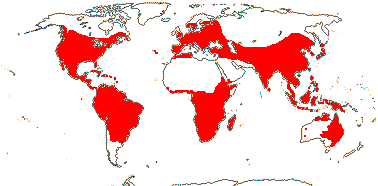
Stem parasites, often ± endophytic°, epicortical roots 0°; (growth in all directions [no polar axis] - Viscum); inositol storage carbohydrates, methylated cyclitols, myricetin, caffeic acid esters, toxic polypeptides +, (mitochondrial Complex I 0 - V., Phoradendron), (chloroplast Complex I [the NDH complex] 0 - V.), essential oils 0, ?santalbic acid; (sclereids +); cuticular epithelium +; stems brittle and jointed°; cuticle waxes usu. platelets with irregular margins; leaves opposite, (members of pairs borne in two ranks), (reduced to scales), lamina with secondary veins usu. ± palmate, (amphistomatous), petiole obscure; plant monoecious° (dioecious - some V.), (flowers perfect - Phacellaria); no bract and bracteole immediately under the ovary°; flower (2-)3-4(-5)-merous°, usu. sessile, small° [4> mm long]; C hairs abaxial to A 0; staminate flowers: C often 4, hairs 0 [?all]; A sessile, anthers opening by pores°/slits, (3-1-locular), (polysporangiate, dehiscence transverse), (connate [= a synandrium], opening circumferentially); (endothecium 0); pollen 3-colporate, (pseudocolpi +), spherical°, surface echinate; carpelate flowers: C often 3, (reduced to apiculae); nectary ± 0; style (solid); ovary with mamelon (0), tracheids 0, 2 "ovules"°; embryo sac bisporic, 8-nucleate [Allium type]/tetrasporic [Adoxa type]/monosporic, 8-nucleate [Polygonum type - V. minimum], (elongated), straight [V.] to U-shaped, starch copious; ("berry" explosive - Arceuthobium, Korthalsella), viscous covering +, incomplete, inside the vascular bundles°, consisting of polysaccharide threads and mucilage°, endocarp +, thin°; endosperm chlorophyllous°, starchy, (plane of first cleavage of zygote vertical - Korthalsella, A.), suspensor short or 0, embryo small to large, chlorophyllous, with 1 cotyledon [= 2 connate], lateral in seed; n = 10-14(-17), nuclear genome [1C] to 100.6 Gb; chloroplast rpl33 gene 0. Note: ° in the description above refers to differences between Visceae and Loranthaceae (at least most of them).
7/520: Phoradendron (235), Viscum (65-150), Dendrophthora (70), Korthalsella (>30). Worldwide. Map: from Barlow (1983: throughout much of the northern Sudano-Sahelian-Zambezian zone), Fl. Austral. vol. 22 (1984) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
Age. An estimate of the age of crown-group Visceae is (135.4-)108.1, 86(-58.1) Ma (Maul et al. 2018).
Evolution: Divergence & Distribution. Visceae probably have been branch parasites for much of the 72 Ma the clade may have existed (Vidal-Russell & Nickrent 2008); the estimate in Maul et al. (2018) would be considerably higher. Arceuthobium is well represented (six species, leaves quite well developed) in Eocene Baltic amber 47-34 Ma (Sadowski et al. 2017a).
The large genus Thesium may be of South African origin - it is certainly mostly African - and it has a stem age of ca 60 Ma and a crown age of (56.5-)42.7-35.9(-24.4) Ma (T. E. Moore et al. 2010: a variety of analyses). Santalum, the sandalwood genus, is centred on Australia and the Pacific, and there have been perhaps two migrations from Australia to Hawai'i and also two migrations out of Hawai'i to the south Pacific (Harbaugh & Baldwin 2007); polyploidy seems often to have occurred before these long distance dispersal events (Harbaugh 2008). Viscum may also have originated in Africa, and Maul et al. (2018) suggests its wide distribution in the Old World may have been aided by the transition from dioecy to monoecy.
Ecology & Physiology. For root parasitism in Santalum album, see Tennakoon and Cameron (2006). Ichihashi et al. (2021) discuss the development of root haustoria in Thesium chinense, noting i.a. intrusive cells at the apex of the haustorium. Overall, there is similaririty with the development of haustoria in Cuscuta (from the stem) and Orobanchaceae (from the roots), q. v..
The branch-parasitic habit seems to have evolved three times in Santalaceae, in Visceae, Santaleae-Eremolepis and Amphorogyneae-Dendromyza (Der & Nickrent 2008; Vidal-Russell & Nickrent 2008).
Press and Phoenix (2005) and Ndagurwa et al. (2016) and references discussed the general ecological effects of the host-parasite interactions of Visceae. Most taxa of Viscum, in particular V. album subsp. album, have broad host ranges (Maul et al. 2018: Fig. 4), although two taxa of Viscum are restricted to conifers and V. minimum to the cactus-like Euphorbia polygona. Korthalsella has a wide host range and shows extensive parallelism; there is strong geographic signal in its phylogeny (Sultan et al. 2019). Most species of the dwarf mistletoe Arceuthobium parasitize Pinaceae, and over 2/3 the records are from Pinus alone, where they can be important pests and have quite high host specificity, while a few species parasitize Cupressaceae (Hawksworth & Wiens 1996; Harrington & Wingfield 1998; Farjon 2008; Mathiasen 2022). Some ex-Eremolepidaceae have a decided preference for Myrtaceae as hosts (Kuijt 1988). Hyperparasitism is quite common. Phacellaria is an obligate hyperparasite on a few other Santalaceae (Dendrophthoë) and especially Loranthaceae, Taxillus in particular (Li & Ding 2006; see also Mathiasen et al. 2008), while Phoradendron durangense and P. falcatum parasitize only other species of Phoradendron (Calvin & Wilson 2009). There are other hyperparasites, "obligate epiparasites", including some ten species of Viscum (see also C. A. Wilson & Calvin 2016; Krasylenko et al. 2021). Somewhat unusually, Arceuthobium neither hosts other parasites nor is an epiparasite - see also Misodendron (Krasylenko et al. 2021).
Watson (2001) suggests that mistletoes s.l. (for which, see above, i.e. including Loranthaceae, are keystone resources for their communities, and gives an example of the interactions between Phoradendron (Visceae) and other plants and animals in the mesquite woodlands in the southwestern United States. Santalaceous mistletoes are are much liked by the great apes (Watson 2001), and Viscum is an indicator of elephant grazing, the animals preferentially eating branches on which it is growing since it is succulent and nutrient-rich, although I doubt that the elephants know much about the latter (Germishuizen et al. 2007 and references: see also Loranthaceae). Similarly, from the Mediterranean eastwards, Arceuthobium oxycedri-infested stems are the preferred forage of sheep and goats, sometimes to the decided detriment of the vegetation in which the parasite grows (Hawksworth & Wiens 1996; see references in Delibes et al. 2017 for the arboreal capabilities of goats).
Some/all species of Viscum and Phoradendron (Visceae) can carry out cellular respiration without mitochondrial Complex I, so ATP production is severely disturbed, and they are the only multicellular organisms known to be able to do this (Schröder et al. 2022). Furthermore, in V. album, at least, the chloroplast lacks chloroplast Complex I, the NDH complex, somewhat less remarkable but with negative effects on ATP production there, too (Schröder et al. 2022). Perhaps the host provides the parasite with energy-rich compounds.
The leaves of Viscum album, at least, fall off the plant when green, senescence not taking place normally (Schröder et al. 2022).
Mistletoes like Viscum and Arceuthobium (also Visceae) have an endophytic stage, and they show early tissue specialization and connection with host xylem, but they have little effect on the anatomy of the host. Species such as V. minimum are basically endophytes even when adult - Tristrix aphyllus (Loranthaceae) is another endophytic mistletoe. They are unlike other endophytic parasites like Pilostyles, Rafflesia, etc., where tissue specialization is late, but this may be associated with the fact that even endophytic mistletoes have some photosynthetic tissue while the others do not (Teixeira-Costa et al. 2021).
Some species of Arceuthobium and Viscum are almost holoparasites, being largely endophytic, the parasite spreading as strands of tissue through the cortex of the host (Nickrent & García 2009; Mauseth & Rezaei 2013; Teixeira-Costa et al. 2020; X. Guo et al. 2021). Arceuthobium depends on its hosts for water and also much photosynthesate, since although the plant, when it finally appears, does contain chlorophyll, it may be as little as 10% of normal amounts; when witches brooms develop, the host becomes particularly severely stressed (Hawksworth & Wiens 1996). Indeed, initial development in Arceuthobium in particular is completely within the host - there are no cotyledons, etc., the epicotylar pole of the embryo aborting, and only the cortical strands of the parasite are evidence of its presence (e.g. Teixeira-Costa et al. 2020). The flow of nutrients from host to parasite is commonly apoplastic (Calvin & Wilson 1996; C. A. Wilson & Calvin 1996). The mistletoe V. minimum, from the eastern Cape, has aerial stems about 3 mm long and with but a single internode (Don Kirkup, pers. comm.); these arise from the endophytic portion of the plant which is found only on the cactus-like Euphorbia polygona (for its growth, see Mauseth & Rezaei 2013). A number of species of Arceuthobium and Viscum have their leaves reduced to sheathing bases, the photosynthesis that does occur being carried out by the stem, and in the latter genus reduction of the lamina is estimated to have occurred ca ten times (Maul et al. 2018). In largely endophytic Visceae there is both a phloic and xylary connection between host and parasite, in the other species, the connection is only through the xylem (Aukema 2003; Tesitel 2016), but the connection is indirect, in the xylary tissues being by tracheidal cells (Teixeira-Costa et al. 2021). At least some species of both Viscum and Thesium (stem and root parasites respectively) take up carbon compounds from their hosts (Giesemann & Gebauer 2019 - see also Loranthaceae, Orobanchaceae).
Finally, a little more about some species of Viscum and Phoradendron, and especially V. album. Some species of Viscum and Phoradendron lack mitochondrial Complex I, so limiting the production of mitochondrial ATP, while in V. album, at least, there is also no chloroplast Complex I, the NDH complex, so further limiting the ATP supply (Schröder et al. 2022). However, V. album obtains carbohydrates like sucrose from its host, and sucrose synthesis uses up much ATP, so the parasite has ways of compensating for its low ATP production. And as Schröder et al. (2022: p. 1905) note of V. album, "It has no polar axis but grows in all directions resulting in the spherical shape of the adult plant; stomata are at both sides of the leaves and not (much) regulated; seed dormancy does not take place; and senescence processes are very reduced as green leaves are discarded". All together, a very odd plant.
Pollination Biology & Seed Dispersal. In Santalum, filament hairs are involved in secondary pollen presentation (Howell et al. 1993), while some Visceae are wind pollinated. In some Santaleae the prominent nectar disc may attract the pollinator (González et al. 2021). Three species of Arceuthobium from the Rocky Mountains were visited by 200+ species of insects, although their flowering times were only somewhat overlapping and their major pollinators (up to six) were also only partly overlapping; pollen was also dispersed by the wind up to 150 m (Penfield et al. 1976). Indeed, pollination here seems particularly unclear/variable, and the female flowers can have large blobs of ?nectar on the stigma - perhaps c.f. pollination droplets in gymnosperms (Hawksworth & Wiens 1996).
Many Visceae, and some Amphorogyneae and Santaleae, mostly stem parasites but including the root parasite Exocarpos, have fleshy fruits and are dispersed by birds, many in a fashion rather similar to the fleshy fruits of Loranthaceae (as "mistletoes" - Watson 2001; Aukema 2003; Watson et al. 2011; Mathiasen 2022). Strings of seeds, "rosaries", from the bird faeces may dangle from the branch (Godschalk 1983; Restrepo 1987: South American species; Reid 1991; see also Mistletoes and Loranthaceae). In Arceuthobium seed discharge is explosive, the seed leaving the fruit at about 1370 cm/second and travelling up to 20 meters (66 feet) before they land, hopefully on a branch (Hinds et al. 1963; Hinds & Hawksworth 1965). Although immediately prior to seed discharge fruit temperature increases substantially because of uncoupled respiration, how that increase might effect seed discharge is unclear (deBruyn et al. 2015). For the dispersal of seeds of some Visceae and Santaleae by birds, see Restrepo et al. (2002), Sultan et al. (2019) and Mathiasen (2022), while Watson (2020 and references) notes that in Madagascar mouse lemurs disperse the seeds around their arboreal dens as they groom their fur, i.e. transport is ectozoic.
Fertilization may be considerably delayed after pollination (Hawksworth & Wiens 1996; Ross & Sumner 2005). In Arceuthobium the seeds can take two years to mature and the embryo lacks a plumule but has a well developed radicle (Ross Friedman & Sumner 2009). Photosynthesis in the green endosperm of Visceae is described as facilitating germination by providing energy for the establishment of the seedling (Tesitel 2016: there is also, for example, green endosperm in the very different Crinum - Amaryllidaceae-Amaryllidoideae).
Plant-Animal Interactions. Mistletoes are a favourite food of primates, and they eat the plants whole (Watson 2001).
Eucalyptus woodland with Exocarpos strictus-dominated understory had about 50% more species of birds than understory dominated by Acacia dealbata, or simply open; the Exocarpos produced fleshy fruits, and also numerous arthropods lived in its foliage (Watson et al. 2011).
Vegetative Variation. Leaf morphology in the old Eremolepidaceae (Santaleae) is very diverse. Thus Eubrachion has peltate scale leaves, while in some taxa what were initially scale leaves resume growth and tip of the expanded leaf is the apex of the scale leaf.
The orientation of cataphylls and their relation to prophylls in genera like Phoradendron (Visceae) as described by Kuijt (1996) are unclear. Similarly, the structures called prophylls by Kuijt (2013) and found on vegetative shoots, as in Arceuthobium azoricum, may well be colleters or something similar, the axillary branches being shown as having their first pair of leaves lateral in position with respect to the axis (= true prophylls?), i.e. in the same plane as these putative prophylls (see also Kuijt 2015: pp. 10-11, esp. Fig. 1d for a discussion). Along the same lines, Ashworth (2000) noted that in Phoradendron, if the first leaves were cataphylls they were in the ad/abaxial plane, while the next pair of leaves, expanded, were lateral whether or not there were cataphylls - but she also noted that there was infraspecific variation in such features. Anatomical/developmental work is needed here.
Genes & Genomes. A genome duplication event (EXCUα) ca 44.8 Ma has been associated with the [Santaleae [Amphorogyneae + Visceae]] clade (Landis et al. 2018). L. N. Rao (1942b: Fig. 11) mentions a "special wall" surrounding the meiosing nucleus on Santalum album, and ingrowths of this wall separate the four nuclei produced.
Molecular evolution has greatly speeded up in the Viscum clade (Vidal-Russell & Nickrent 2008). In Viscum, alone of Santalales examined, the genome is very large, the 2 C value being some 205.8 pg or more, Viscum album having the largest genome (Leich et al. 2005; c.f. Zonneveld 2010). The amount of DNA per chromosome is perhaps the highest in all eudicots (Jordan et al. 2014; Hidalgo et al. 2017c). See Wiens and Barlow (1971) for cytology.
Petersen et al. (2015) looked at the plastomes of Osyris (Santaleae) and three species of Viscum, and found that the latter had rather smaller plastomes, especially evident in the small single-copy region, and that all had lost the eleven genes coding for the NAD(P)H-dehydrogenease complex (or they were pseudogenized), as in other parasites and mycoheterotrophs - and some other groups, as well (see also X. Chen et al. 2019: missing in all santalalean hemi/holoparasites they examined). Viscum minimum, although much reduced vegetatively, had a plastome not that dissimilar from that of the other species in the genus examined. X. Guo et al. (2021) discuss similar plastome reduction in Arceuthobium, although Schneider et al. (2021) found little pseudogenization or gene loss here, apart from missing cemA. For further details of plastome variation, see Chen et al. (2019).
Viscum scurruloideum, not a notably reduced species, has a tiny mitogenome (ca 66 kb) with high substitution rates, while V. album, on the other hand, has a far larger mitogenome of ca 565 kb, a remarkable difference; V. scurruloideum has the fewest protein-coding genes - 19 - of any angiosperm known, and it has lost its respiratory complex I, pratically unique in eukaryotes, yet at the same time it has remarkably large repeat pairs that are practically identical (Skippington et al. 2015, 2017: c.f. size variation in Silene). These and at least some other species of Viscum lack all 9 nad genes, genes that code for the first enzyme in the mitochondrial electron transport chain that is involved in respiration, and also other related genes - a few unicells are the only other organisms that lack this complex, but it is present in all other land plants (Maclean et al. 2018). Oxidative phosphorylation is much reduced, although the rest of the mitogenome is rearranged and some respiration does occur (see also Skippington et al. 2017; G. Petersen et al. 2020). However, Viscum is a slow-growing plant and may have little need for much mitochondrial ATP, and ATP seems to be produced by glycolysis rather than mitochondrial respiration (Senker et al. 2018; Maclean et al. 2018). Most other mitochondrial genes in these plants are highly divergent compared with those of other land plants and have much elevated synonymous and non-synonymous substitution rates, most appearing to evolve under relaxed selection (Petersen et al. 2020 and references). Reduced respiration is known from some animal parasites, too (Santos et al. 2018), but the mitogenome of other parasitic and of free-living plants is normal (Maclean et al. 2018); it is unclear if the loss of mitochondrial genes here is connected with parasitism (Petersen et al. 2019, 2020). Substitution rates are high in other species of Viscum like V. album, and in this case there has been lateral gene transfer from a host in Ericales (matR, from a plant in the Diapensiaceae-Ericaceae area) and in other Santalales (ccmB: Loranthacaeae, perhaps close to Amyema), the latter gene probably moving during a hyperparasitism event (Skippington et al. 2017).
Economic Importance. Mathiasen et al. (2008) provide a list of Santalaceae that harm crops and timber trees (see entries in . Species of Arceuthobium (e.g. A. americanum) are major pests on conifers in west North America in particular, and they cause extensive witches' brooms and the ultimate death of the host; the amount of timber lost is substantial, some 15.1 million m3 per year in the U.S.A. and Canada alone (e.g. Unger 1992; Hawksworth & Wiens 1996; Sadowski et al. 2017). See also entries in the Invasive Species Compendium (CAB International).
Chemistry, Morphology, etc.. The pith of twigs of Viscum album is like an eight-pointed star (IAWA J. 23(1) - Cover). The cuticle in Visceae becomes progressively thicker and epidermal cells may die and get incorporated into this cuticular layer (it may be close to 600 μm across - Damm 1901), some subepidermal cells may divide, but there is no cork cambium/cork - indeed, the cuticular layer may be thicker than the cork on tree stems of equivalent thickness (Damm 1901; C. A. Wilson & Calvin 2003). This is the cuticular epithelium, and it lacks both suberin (c.f. cork) and lenticels (Wilson & Calvin 2003); it is present in Eremolepidaceae (Santaleae), some other Santaleae, and Visceae, but its distribution needs clarification.
For inflorescence morphology in Visceae, see e.g. Kuijt (1959), Suaza Gaviria et al. (2017), Nickrent et al. (2019), and references. To say that inflorescence vasculature can be difficult to interpret is an understatement, as the illustration of that of Dendrophthora flagelliflorus by Kuijt (1959) suggests, although relatively little work seems to have been done in this area (but see York 1913). A number of Santalaceae have three traces in their perianth members, the two lateral traces coming from commissural bundles (F. H. Smith & Smith 1943), but Visceae, for example, have but a single trace, and the stamens may even lack traces (Kuijt 1959). González et al. (2021) looked the development of the highly reduced and unisexual flowers of Antidaphne viscoidea (Santaleae) in some detail; they found petals, although some reports suggested they were absent; they also discuss the massive nectariferous disc, monosymmetry, etc.. Note that I do not distinguish between perfect and unisexual flowers in the characterization of Santaleae; González et al. (2021) did not find staminodes in female flowers or pistillodes in male flowers.
Ovule, embryo sac and embryo development of many plants in this clade are all more or less remarkable; for further discussion, see above. Although the embryo in the Cervantesia group is close to half the length of the seed, being slender it looks tiny (Rogers et al. 2008).
See Hawksworth and Weins (1972, 1996) for Arceuthobium, Kuijt (1988: Eremolepidaceae, 2003: Phoradendron), Stauffer (1969: Amphorogyneae) and Rogers et al. (2008: Cervantesia group, new genera), also Solms-Laubach (1867), L. N. Rao (1942a: Santalaceae) H. C. Weber (1984) and Benzing (1990) for haustorial anatomy, growth, etc., of stem parasitic taxa, Norverto (2004, 2011) for wood anatomy, Swamy (1949c) and C. A. Wilson and Calvin (2003) for some aspects of anatomy (the former also for pollen), Leins (2000) for floral morphology of Viscum, Ronse de Craene and Brockington (2013) for flowers of Colpoon, Feuer and Kuijt (1978) and Feuer et al. (1982) and references for pollen, van Tieghem (1869a: Viscum), Treub (1882: Viscum), Guignard (1885: Thesium, Osyris, Santalum), Steindl (1935: Viscum), Rutishauser (1937), Schaeppi and Steindl (1937: Osyris), Rao (1942b: Santalaceae), Ram (1957: Comandra, 1959: Exocarpos), Paliwal (1956), Joshi (1960: Osyris), Manasi Ram (1960: Leptomeria), Bhatnagar (1968: Mida), Bhatnagar and Agarwal (1961: Thesium), Bhandari and Vohra (1983), Zaki and Kuijt (1995) and Ross and Sumner (2004, 2005) and references for embryology, and Kuijt (1982) for embryos of Eremolepidaceae.
Phylogeny. Der and Nickrent (2005, esp. 2008; see also Nickrent et al. 1998) found seven well supported clades in Santalaceae (see the groups above), but relationships between these clades were poorly understood; there may be a clade including most of the family except the Cervantesia, Thesium and Comandra clades. There is very slight support for a [Cervantesia group + Thesieae] clade, and some support for a [Amphorogyneae + Visceae] clade, etc.; the last clade appeared in both plastid and non-plastid analyses in Su et al. (2015), but again, other relationships were unclear. In the recent genus-level Santalales-wide analysis by Nickrent et al. (2019) that used two nuclear and three chloroplast markers, the relationships obtained were [[Cervantesia group [Thesieae + Comandra group]] [Santaleae [Nanodea group [Amphorogyneae + Visceae]]]], in part supporting relationships that were becoming apparent in the earlier analyses. Z. Zhou et al. (2019) using two nuclear and two plastid markers (the same as in Nickrent et al. 2019) recovered a topology [[Comandra group [Cervantesia group + Thesieae]] [Nanodea group [Santaleae [Amphorogyneae + Visceae]]]] - something is odd here. Finally, when Nickrent (2020) combined Su et al. 2015 with Nickrent et al. (2019) he suggested the relationships [[Thesieae + Comandra group]] [Cervantesia group [Santaleae [Nanodea group [Amphorogyneae + Visceae]]]].
Thesieae. Nickrent et al. (2008), Moore et al. (2010), Nickrent and García (2015), Zhigila et al. (2020) and especialy Garcí et al. (2024: ca 230 sp., 196 named; nrDNA ITS and chloroplast trnLF and trnDT) examined relationships within the large genus Thesium. Zhigila et al. (2020) in particular noted fairly extensive conflict there in the topologies of trees produced by the analysis of nuclear and plastome genes, and Garcí et al. (2024) recorded "remarkable incongruences" betwen ITS and chloroplast trees at the specific and even infra-specific levels, especially in the Cape subgenus Frisea - perhaps horizontal gene transfers.
Santaleae. Genera of the old Eremolepidaceae have very often been kept separate before, but they are well embedded in the Santalum clade (Der & Nickrent 2008), their strongly supported relationships being [Antidapne [Lepidoceras + Eubrachion]]. See Harbaugh and Baldwin (2007) for a discussion of the phylogeny of Santalum, the sandalwood genus.
Visceae. Nickrent et al. (2004b: ?rooting) discuss relationships in Arceuthobium; Schneider et al. (2021) recovered reciprocally monophyletic Old and New World clades here and congruent nuclear and plastome phylogenies. Ashworth (2000, 2017) examined relationships around Phoradendron, which is probably not monophyletic (Dendrophthora is muddled up with it, see also Maul et al. 2018). Relationships in Viscum have been considerably clarified by Maul et al. (2018). Phylogenetic relationships in Korthalsella conflict with previously-recognized infrageneric groupings, and species limits there also need much attention (Sultan et al. 2019).
Two groups, both stem parasites, are morphologically rather distinctive:
Classification. Der and Nickrent (2005) proposed that all major clades in Santalaceae should be recognized as families; the seven families listed there are the necessary result if Viscaceae are kept separate (see especially Nickrent et al. 2010; also Su et al. 2015). However, apart from Visceae and Amphorogyneae, distinctive features for the clades are elusive. Indeed, much of the character hierarchy above is rather notional, moreover, relationships within the family are rather unclear.
Zhigila et al. (2020) proposed an infrageneric classification for Thesium, and they recognized five subgenera, however, Garcí et al. (2024) suggest that a number of changes will be needed there.
Previous Relationships. Both Cronquist (1981) and Takhtajan (1997) recognized Eremolepidaceae (now in Santaleae), Viscaceae and a quite broadly delimited Santalaceae, the latter also including some Schoepfiaceae in his Santalaceae. Eremolepidaceae also gave Kuijt (1968) some trouble; he thought that they were perhaps close to Misodendraceae, not to Viscaceae (= Visceae), and he toyed with the idea that Lepidoceras was better placed in Loranthaceae.
Botanical Trivia. Viscum crassulae is apparently a pleasing horticultural subject with its bright red fruits; it must be about the only stem parasite so grown.
Sarcopodum (= Exocarpus) was described as a gymnosperm family.