EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent (ca 2019 onwards) work suggests a polytomy is probably the best way to visualize relationships around here. For further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan - Back to Main Tree
Woody*; nicotinic acid metabolised to its arabinosides; (iridoids +); ectomycorrhizae 0; tension wood decidedly uncommon; leaves simple*; flowers perfect*; C enclosing A and G in bud, free*; anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; fruit ?; seeds exotestal [see below], cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm long, cellular. - 20 orders, 103 families, 5,271 genera, 1,000,593 species.
Note: The characterization above provides a bit more detail - including some probable plesiomorphies, which have asterisks - than in similar cases elsewhere on this site. This is because the immediate relatives of asterids are unclear.
In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. Note that the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The age of crown-group asterids has been estimated to be ca 128 Ma, mid Early Cretaceous (K. Bremer et al. 2004a), (128-)126(-122) Ma by Wikström et al. (2015) or (140.8-)125.4(-109.4) Ma by Tank and Olmstead (pers. comm.); Wikström et al. (2003: c.f. topology) suggest an age of (122-)117, 107(-102) Ma (117-107.8 Ma in Schenk & Hufford 2010), Janssens et al. (2009) an age of ca 128 Ma, while Anderson et al. (2005: only Cornales and Ericales sampled) give an age of ca 109 Ma. Soltis et al. (2008) suggest ages of 130-115(-86) Ma, Magallón and Castillo (2009) ca 106.35 Ma, Sun et al. (2013) 104-99 Ma, and Magallón et al. (2015) ca 114.6 My. Other estimates range from around (122-)114, 106(-100) Ma (Bell et al. 2010), 113-104 Ma (Zeng et al. 2017), 135.9-132.4 Ma (Nylinder et al. 2012: suppl.), 167-103 Ma (Schneider et al. 2004), (103-)93(-81) Ma (N. Zhang et al. 2012), ca 112.5 Ma (Fu et al. 2019a), ca 113.2 Ma (X. Guo et al. 2021), ca 121.4 Ma (C. Zhang et al. 2020), 99-88 Ma (Jiao & Wang 2022), or as old as ca 141 Ma (Z. Wu et al. 2014) and 160-117 Ma (Barba-Montoya et al. 2018) - or as young as (89-)84(-80) Ma (Moore et al. 2010: 95% HPD).
Martínez-Millán (2010) evaluated the asterid fossil record, which for the most part is not very rich (the Cornaceae-Alangiaceae area is one notable exception), and provided a series of fossil-based ages for asterid clades, with asterids going back to the Late Cretaceous ca 89.3 Ma (Turonian), with Cornales, Ericales, and lamiids and campanulids/asterids I and II all being found in the fossil record by ca 83.5 Ma (Late Santonian-Early Campanian). There are reports of earlier fossils, including Eoëpigynium burmensis, from 110-97 Ma (Poinar et al. 2007; Poinar 2011 - flowers 4-merous, perhaps Cornales, but also perhaps Saxifragales, Myrtales, Asterales..., K quite well developed) and fossils of some 124 Ma age that are perhaps Sarraceniaceae (Ericales) (Li 2005). Finally the Late Cretaceous Scandianthus (Friis & Skarby 1982) shows phenetic similarity with Vahliaceae, Hydrangeaceae, Phyllonomaceae, Escalloniaceae, and even some Saxifragaceae s. str. (Friis et al. 2011). These earlier fossils are difficult to integrate into the phylogeny.
Evolution: Divergence & Distribution. For a review of the fossils attributed to asterids, see Martínez-Millán (2010); the focus is on how the papers reporting these fossils presented their data. See also Manchester et al. (2015).
Note that there are many ages for nodes within asterids that may not appear in the pages here since they have come mostly from analyses of plastome genes that have been rendered suspect because of the topological changes that are resulting from analyses of nuclear genomes. Wikström et al. (2015) provide dates throughout the asterids, and they tend to be younger - e.g. 3-16(-22) (Dipsacales) Ma younger (see their Table 3, but not Gentianales) - than those in K. Bremer et al. (2004a); Wikström et al. (2015) suggest that this is largely because of the taxa included, how they calibrated the root node and how they treated divergence rates. C. Zhang et al. (2020) compare their ages with those of these authors.
Thinking about asterid evolution in the context of Cretaceous history is of course the next step. Bremer et al. (2004a) thought that Cornales and Ericales diverged soon after the origin of the stem group asterids ca 128 Ma, mid Early Cretaceous, and the other asterid orders had all diverged before 100 Ma, i.e. in the Early Cretaceous by the middle of the Cretaceous Terrestrial Revolution - for which, see elsewhere. Wikström et al. (2015) found that much of the divergence within the campanulids, but not in the other major groups, was in the Late Cretaceous, and that in general family divergences were in the Late Cretaceous. C. Zhang et al. (2020: p. 11) noted that divergences along the asterid spine (the quick separation of 10 clades) happened during a brief period of a "dramatic" (but otherwise unspecified) climate shift that began ca 120 Ma, but the origin of the bulk of the asterid orders occured up to 10 million years or so after this period; family-level diversification had largely occured by the end of the Cretaceous. Of course, this is where rank can obscure rather than clarify what is going on. From Fig. 6 in Zhang et al. (2020) it seems that families in Boraginales separate notably later, on into the Eocene (but some would argue that the whole lot should be a single family, indeed, the whole order, with around 3,100 species, is only moderately speciose). Asteraceae separate from Calyceraceae towards the end of the Palaeocene (of course major diversification in Asteraceae does not happen until considerably later still...). Although the asterids do have a number of distinctive features, it is clear changes somewhat after the euasterid node, certainly after this node, indeed more within Gentianales, Asterales, even within Asteraceae, and so on, are likely to have driven asterid diversification - at least in terms of species numbers.
Endress (2011a) suggested that a key innovation for a clade [Ericales + core Sympetalae] was sympetaly. There is extensive variation in corolla development in Ericales and Cornales in particular, and indeed also in the ex-Icacinalean woody clades at the base of the lamiids and campanulids (see elsewhere). Sympetalae of older studies were defined largely by their sympetalous corolla, but some families here included in the asterids, perhaps particularly in some of the basal clades, seem to be polypetalous. However, developmental studies like those of Erbar (1991) note that at least some apparently polypetalous taxa have a ring primordium very early in development (see, for example, Reidt & Leins 1994), i.e., they show early sympetaly. (It is somewhat paradoxical that this early corolla tube formation should quite often be associated with a corolla that appears to have separate petals at maturity!) However, the position of early initiation of the corolla tube on the tree is quite uncertain. Apiales, Asterales, and Dipsacales have many members with such initiation, as do both Oleaceae and Rubiaceae, basal or almost so in their orders in the lamiids, and also some Cornales (see Erbar & Leins 2011 for a recent survey). Sampling still leaves much to be desired, but the condition of early initiation could conceivably be a synapomorphy for the asterids (see Erbar & Leins 1996b; Leins & Erbar 2003b for more details), even if the petals are functionally ± free, at least at anthesis. There may be an association between early corolla tube formation and flowers with an inferior ovary (Ronse Decraene & Smets 2000) and families like Oleaceae with superior ovaries and apparently early corolla tube formation need more study from this point of view; the character needs re-evaluation. Only in a number of Ericales and core asterids/gentianids does the mature flower have a decided corolla tube, hence the tentative assignment of posession of an obvious corolla tube as an apomorphy for [Ericales + other asterids] in pre 2020 versions - but see Stull et al. (2018); taxa with a pronounced corolla tube quite often have late corolla tube initiation, the petal primordia initially being free. Zhong and Preston (2015) discussed the development of sympetaly, distinguishing between the lower corolla tube, which comes from the elongation of common petal/stamen bases, and the upper corolla tube, often with postgenital fusion.
C. Zhang et al. (2022: p. 3188) described the ancestral condition for asterids as being a "woody terrestrial plant with simple leaves, bisexual, and actinomorphic flowers with free petals and free anthers, a superior ovary with a style, and drupaceous fruits" - all plesiomorphic features apart perhaps for the drupaceous fruits. K. Bremer et al. (2001), Stull et al. (2018) and Zhang et al. (2020) suggest morphological synapomorphies for asterids and some groupings within them. Gasser and Skinner (2018) suggested that the euasterid clade was characterised by ovules with a single integument (see also Endress ); this character is provisionally plced here. In general, where many characters are to be placed on the tree depends on resolution of relationships within and between Ericales and Cornales, and even then the pattern of gain-loss of some of these features is liable to be complex (Stull et al. 2018).
Floral variation in particular in Ericornids is quite considerable, and as mentioned elsewhere features found in more basal Pentapetalae are found here, but only rarely, if at all, in other asterids in the gentianid clade. Some characters common in asterids, including those of wood anatomy, probably have functional and logical linkages that also must be taken into account; for a survey of wood anatomy of Sympetalae in the old sense, see Carlquist (1992b). Thus the presence of a tenuinucellate nucellus is linked with that of unitegmic ovules (see also Erbar & Leins 2011), the development of an endothelium (Kapil & Tiwari 1978), and a simple exotestal seed type (but see below: Netolitzky 1926); that of sympetalous monosymmetric flowers with epipetalous stamens, etc.. Ericornids also show much variation in chemistry, degree of sympetaly, stamen number and development, adnation of stamens to corolla, and in ovule morphology and anatomy as is found in rosids, Dilleniales, etc.. Thus members of Ericornids may have ellagic acid; interestingly, they contain the only families in which both iridoids (common in the euasterids) and ellagic acid (common outside the asterids) occur (Cornaceae, Symplocaceae, Ericaceae and Fouqueriaceae - Bate-Smith 1984). Geraniol synthase is involved in an early step in iridoid synthesis, diverting resources that might otherwise be used in monoterpene synthesis into iridoids, which have been described as "non-canonical monoterpenes" (Boachon et al./Mint Evolutionary Genomics Consortium 2018). For possible additional synapomorphies in this area, see Chemistry, Morphology, etc., below.
Ecology & Physiology. Leaf size increases and plant height shows a notable decrease at this node (Cornwell et al. 2014), although the former character subsequently decreases both in Ericaceae and in the gentianids.
Iridoids, common in asterids, have been implicated in herbivore preferences, deterring some and attracting others (e.g. see discussion under Plantaginaceae, Scrophulariaceae, etc.: Bowers 1980, 1988, 1993; Smilanich et al. 2009; trade-offs involving iridoid sequestration; Dobler et al. 2011). Thus iridoid glycoside abundance was higher in trees of Fraxinus excelsior susceptible to the the ascomycete Hymenoscyphus fraxineus, but such trees may also be less susceptible to herbivores (Sollars et al. 2017), indeed, iridoids may protect plants against bacterial and fungal pathogens under other circumstances (Dobler et al. 2011 and references). Iridoids have a bitter taste and are emetics for vertebrates, at least; overall herbivory in asterids is relatively low (Turcotte et al. 2014: see caveats). Iridoids may also be involved in plant-plant relationships. Parasite Orobanchaceae may produce toxic iridoid aglucones in their hosts and so increase their effect on the latter (Rank et al. 2004), while iridoids from roots of Verbascum (Scrophulariaceae) may depress germination of competitors (Pardo et al. 2004). Volatile iridoids, such as those found in Lamiaceae-Nepetoideae, may have yet other functions (Boachon et al. 2018).
Maherali et al. (2016) noted that there were very few records of ectomycorrhizae in the superasterids, i.e. asterids along with Caryophyllales, Santalales and Berberidopsidales. This may be connected with the fact that the herbaceous habit is common here - although by no means universal.
See Batashev et al. (2013 and literature) for the anatomy of minor-vein phloem (the typology is complex) and its physiological implications.
Plant-Animal Interactions. Pentzold et al. (2014) discuss how insects can get around iridoid defences. Iridoids are sometimes sequestered by the insect eating the plant and used in its own defence against predators (Bowers 1993; Dyer & Bowers 1996; Nishida 2002 for a summary), and it is interesting that it is nearly always aucubin or catapol that are sequestered (Dobler et al. 2011) - confusing the issue, iridoids may also be synthesized de novo by the insect (Burse et al. 2009: Chrysomelina).
Insect preferences can be striking: Uraniidae (moths) are found on Dipsacales, Lamiales, Gentianales - and also Daphniphyllaceae, an iridoid-containing member of Saxifragales (Lees & Smith 1991), while larvae of Nymphalidae-Melitaeini butterflies are also almost restricted to asterids, although they are also quite common on Asteraceae and Acanthaceae, which, although asterids, lack iridoids; Melitaeini distinguish between plants with route I secoiridoids, which they eat, and route II decarboxylated iridoids, which they rarely eat (Wahlberg 2001).
Genes & Genomes. C. Zhang et al. (2020) noted numerous WGDs is the asterids, some 34, all but four of which were within families and of which 17 were new; O.T.P.T.I. (2019) had recognized 41 duplications, of which 18 were new. Of the 27 WGDs in O.T.P.T.I. (2019) that had 2≤ representatives in the analysis of Zhang et al. (2020), 23 were also recognized by this latter group. A reminder: duplications within families are often not mentioned here.
Chemistry, Morphology, etc.. Albach et al. (2001a) discussed iridoid distribution, etc., in the asterids, as do Soltis et al. (2005b). Mølgaard and Ravn (1988) and Rønsted et al. (2002) outlined the systematic utility of caffeic acid derivatives; chlorogenic acid, an ester of caffeic and quinic acid, is especially common in asterids, but also occurs elsewhere (see also also Lamiales and Boraginaceae in particular for other derivatives).
Characteristic of the whole clade - although with numerous exceptions (derived), is the Baileyan wood anatomical syndrome of predominantly solitary vessels, scalariform perforation plates, mainly opposite vessel pitting, very long vessel elements and fibres at least 800 and 2190 µm long respectively, non-septate fibres with distinctly bordered pits, and diffuse to diffuse-in-aggregates and scanty paratracheal axial parenchyma (Lens et al. 2008). Compound leaves are relatively uncommon in asterids, and when they occur the leaflets are often not articulated and/or distinct (but c.f. Araliaceae!), however, elements of development are largely identical in very different-looking compound leaves (Bharathan et al. 2002; Blein et al. 2008). Taxa with stipules are also fairly uncommon.
Taxa with apetalous flowers are uncommon in asterids, as are taxa with a tube-forming hypanthium (c.f. in rosids). Monosymmetry may have arisen some fifteen times here, with several reversals in Lamiales and Dipsacales (Jabbour et al. 2008: see also Donoghue et al. 1998; Ree & Donoghue 1999); monosymmetric flowers may have one, or rarely two, spurs (Jabbour et al. 2008).
For a discussion on corolla tube development, see below. Gasser and Skinner (2018) suggested that the euasterid clade - but here asterids as a whole = was characterised by ovules with a single integument that were derived from the bitegmic condition by adnation of the integuments (see the KANADI gene ABERRANT TESTA SHAPE). As might be expected if there is adnation of two integuments, the single integument that results is often notably thick. Furthermore, in bitegmic ovules the outer integument is involved in the flexion of the ovule, and anatropous ovules are common here; the absence of the outer integument, as when the INNER NO OUTER gene becomes non-functional, is often associated with straight/orthotropous ovules (Gasser & Skinner 2018).
The direction of contortion in flowers with contorted petals tends to be consistent - again, c.f. rosids, where it may be labile even within an individual (Endress 1999, 2001b, 2010c)), although exactly where the switch might occur on the tree is unclear. Lee et al. (2004) suggest that the CRABS CLAW gene is expressed in the rather different nectaries in the rosids (receptacular nectary) and asterids (gynoecial nectary) that they sampled; Bernadello (2007) surveyed nectary variation in asterids.
Endress (2010c) noted that ovules in this clade are frequently unvascularized, although the exact distribution of this feature is unclear; taxa with vascularized ovules are also quite common (e.g. Guignard 1893). The integument, when single, is often dermal in origin, as is the inner integument of other angiosperms, while the outer integument is largely subdermal (but c.f. monocots: see Bouman 1984; de Toni & Mariath 2009 - I have not looked at this character in detail). A suggestion might then be that the single integument of asterids corresponds to the inner integument of many bitegmic angiosperms, but since a number of Cornales and Ericales in particular have bitegmic ovules, the story is unlikely to be simple. Indeed, the nature of the single integument so common here has occasioned much speculation, and it may well be a composite structure (Bouman & Calis 1977; Kelley et al. 2009; McAbee et al. 2005; Endress 2011b; Lora et al. 2015). Many asterids also have anatropous ovules, and curvature of the ovules in other angiosperms is commonly associated with the presence of a second integument (Endress 2011b). In the characterizations below, the asterid seed coat is described as being testal, although the term "testa" technically refers to that part of the seed coat that is derived from the outer integument. Commonly only the outer epidermal layer of cells is thickened and lignified, and this mostly on the anticlinal and the inner periclinal walls. This type of seed coat was called the Ericaceous-type seed by Huber (1991), and he emphasized its wide distribution; note that Caryophyllales have an strictly exotestal seed. Arillate seeds are decidedly uncommon in asterids, in part because the seeds are frequently very small.
Phylogeny. See the Dilleniales page for a discussion on the relationships of the asterids. I used to think that Caryophyllales or Santalales (or the two as a combined clade) might be their sister group, but the trees in C. Zhang et al. (2020) and W. J. Baker et al. (2021: see Seed Plant Tree) suggest otherwise - perhaps Berberidopsidales are in that position.
Asterid monophyly was early well established (e.g. Olmstead et al. 1992, 1993, 2000; P. Soltis 1999); Albach et al. (1998) suggested the four main groupings recognised here. Relationships in phylogenies proposed by K. Bremer et al. (2001: analysis of 2 genes + morphology) and Albach et al. (2001b: analysis of four genes) are largely congruent. Differences were almost entirely in taxa not assigned to orders by A.P.G. (1998), although many of these have since found a home, relationships in the provisional Bayesian analyses of Lundberg (2001b, d) pointing the way to many of those currently accepted. B. Bremer et al. (2002) provide an early comprehensive phylogeny of the clade, although with minimal sampling within families, using three coding and three non-coding chloroplast markers. Both B. Bremer et al. (2003) and Olmstead (2000) suggested that there was strong support for Cornales being the sister to all other asterids; see also Albach et al. (2001), Soltis et al. (2003), J. Li and Zhang (2010), H.-T. Li et al. al. (2021), etc.. Although Hilu et al. (2003) reversed the positions of Cornales and Ericales, they sequenced the matK gene alone; Caiophora (Loasaceae) appears in Asterales, far separate from the other members of the family - a case of mistaken identity? There are other suggestions. Thus Qiu et al. (2010: four mitochondrial genes, support mercifully poor) found Ericaceae to be sister to a clade [paraphyletic Cornales + rest of asterids] while Lee et al. (2011) even found Vaccinium to be sister to Caryophyllales in some analyses, with Cyclamen sister to Panax... Tank and Donoghue (2010) provide a largely resolved tree of relationships between asterid orders, and the topology of basal asterid relationships here was for some time the same as theirs (Fiz-Palacios et al. 2011 have additional suggestions). For trees produced by analyses of 18S/26S nuclear ribosomal data, see Maia et al. (2014); very few deeper relationships have much support. C. Zhang et al. (2020: various analyses, data sets with 387-1769 genes) looked at 365 asterids - there were representatives of all orders and 102 of the 110 families then recognized. Details of the relationships that they found are mentioned in various places over the remaining order pages, but it should be mentioned here that Zhang et al. (2020) found that Aquifoliales, Bruniales and Icacinales were polyphyletic, the latter turning up in three places; the polyphyly of Aquifoliales and Icacinales is associated with the reconfiguration of relationships at the base of the Campanulids and Lamiids that has been happening over the last few years largely caused by the use of nuclear data.
The phylogenetic position of Hydrostachyaceae, a highly-modified aquatic herb, has long presented problems, and some have already been discussed above. The embryology of the family shows some similarities with that of Crassulaceae, but relationships neither there nor with Podostemaceae (see below) can be maintained given what we now know about relationships of these clades. Members of sympetalous groups, especially Lamiales, show some similarities to Hydrostachyaceae in ovary structure (apical septae) and in ovule and endosperm development. However, although the coenocytic micropylar haustorium is well developed, the chalazal endosperm cell, which remains undivided, is barely haustorial, and the two carpels are collateral, rather than superposed as in most Lamiales (Jäger-Zürn 1965; see also Rauh & Jäger-Zürn 1966, 1967 [strongly support for a relationship with Lamiales]; Leins & Erbar 1988, 1990). However, in some Orobanchaceae, for example, the chalazal haustorium is also very poorly developed (Tiagi 1963), as in Lamiales basal to Calceolariaceae. A position within Hydrangeaceae has also seemed to be quite likely (Xiang 1999; see also Hempel et al. 1995; Olmstead et al. 2000; Albach et al. 2001; Wikström et al. 2001; Fan & Xiang 2001; Xiang et al. 2002; Bell et al. 2010 - even in Xiang et al. 2011 this position cannot be excluded), but note that Hydrostachyaceae have a very long branch; what about the mitochondrial coxII.i3 intron (Joly et al. 2001)? As Albach et al. (2001) observed, few morphological characters support this position, but then one could argue that this is perhaps to be expected of any highly-derived aquatic plant. Schenk and Hufford (2010: support weak) and Magallón et al. (2015) placed Hydrostachyaceae as sister to all other Cornales. Although Hydrostachyaceae were not included in the plastome analysis of H.-T. Li et al. (2019), in Li et al. (2021), relationships (well supported) were [Nyssaceae [Hydrostachyaceae [Hydrangeaceae + Loasaceae]]] (see also below).
In a five-gene analysis Burleigh et al. (2009) found that there was strong support (97% ML bootstrap) for a position of Hydrostachys within Lamiales, largely because of the matK sequence added. Where in the Lamiales Hydrostachys might be placed was unclear, although it would probably be in a clade that excluded Oleaceae, at least. More comprehensive analyses are needed; Calceolaria and other clades below it in Lamiales other than Oleaceae were not sampled. Indeed, although morphologically Hydrostachyaceae are more or less at home in Lamiales (and I initially thought that they might end up there), more comprehensive analyses (Schäferhoff et al. 2010) exclude them from that order; a sequence used by Burleigh et al. (2009) was similar to that of Avicennia (Acanthaceae)... However, the focus of the work by Schäferhoff et al. (2010) was on relationships within Lamiales, and they did not place Hydrostachyaceae with confidence. Rather remarkably, W. J. Baker et al. (2021a: see Seed Plant Tree) recently found that Hydrostachys, the only Hydrostachyaceae included, was sister to all other asterids (its position remained the same in Version 2 of this tree, Jan. 2022), although at this time S. K. Thomas et al. (2021) placed it sister to [Hydrangeaceae + Loasaceae], albeit with some reservations (see below), and recently Nguyen and Atkinson (2024) found a clade [Loasaceae [Hydrostachaceae + Hydrangeaceae]], but this part of Cornales was not the focus of their work. Z. Xu et al. (2024: 338 single-copy nuclear genes and 78 chloroplast genes) found Hydrostachyaceae to be sister to [Loasaceae + Hydrangeaceae] in various analyses (Grubbiaceae and Curtisiaceae not included), however, there were clearly problems with MatK, Hydrostachyaceae apearing in three quite different places in core eudicots in MatK analyses. Zuntini et al. (2024) and as of 1.viii.2024 it is not one of the some 10,700 or so specimens in the Tree of Life at all...
There have been suggestions that Ericales and Cornales formed a clade. Thus Morton (2011: nuclear Xdh gene) found some support for an [Ericales + Cornales] clade, but sampling in the latter was poor (see also N. Zhang et al. 2012: weak support, nuclear genes; Zeng et al. 2017: not the focus of their study). Indeed, in recent analyses using nuclear genomes - see O.T.P.T.I. (2019), Stull et al. (2020a) and particularly C. Zhang et al. (2020) and W. J. Baker et al. (2021a: see Seed Plant Tree) a [Cornales + Ericales] clade has consistently been recovered, and usually with strong support; Zhang et al. (2020) named this clade the Ericornids (see also S. K. Thomas et al. 2021). Stull et al. (2020a) toyed with the idea that there had been ancient hybridization between Cornales and some gentianid/core asterid, or perhaps incomplete lineage sorting, that resulted in the sister group relationship between these two orders, and they found that at the level of individual genes (83 examined), slightly under two thirds were concordant with the Ericornid group. In any event, this clade is recognized below (see also Stull et al. 2023). There is strong support for the Ericornids in Zhang et al. (2020).
Previous Relationships. The distinction between all other angiosperms and the asterids partly corresponds to the distinction between the crassinucellate and tenuinucellate groups of Young and Watson (1970: phenetic analyses). However, there are also substantial differences, for example, Young and Watson included Apiaceae-Araliaceae in their crassinucellate group. Philipson (1974) further emphasized the distinction between the crassinucellate and tenuinucellate groups of Young and Watson, linking the two via Celastraceae, Grossulariaceae and Brexiaceae (here Celastrales, Saxifragales, and Crossosomatales, all currently placed in rosids and so not immediately related to asterids); Theales, Primulales and Ebenales together made up a separate lineage (here part of Ericales). Later Philipson (1977) resurrected van Tieghem's (1901) names Unitegminae and Bitegminae for these two groups; integument number, nucellus condition and ovule curvature are correlated. General morphology indeed suggests such relationships, as Hufford (1992a) found with morphological phylogenetic analyses - Theaceae, Paracryphiaceae, Apiaceae and Araliaceae were members of Rosidae (but Pittosporaceae were sister to Polemoniaceae).
Synonymy: Aquifolianae Doweld, Aralianae Takhtajan, Asteranae Takhtajan, Balsaminanae Doweld, Boraginanae Doweld, Brunianae Doweld, Campanulanae Reveal, Cornanae Reveal, Diapensianae Doweld, Dipsacanae Takhtajan, Ericanae Takhtajan, Escallonianae Doweld, Eucommianae Reveal, Gentiananae Reveal, Lamianae Takhtajan, Lecythidanae Reveal, Loasanae Reveal, Oleanae Takhtajan, Phellinanae Doweld, Primulanae Reveal, Solananae Reveal Sarracenianae Reveal, Theanae Reveal, Vahlianae Doweld - Asteridae Takhtajan, Cornidae Reveal, Ericidae C. Y. Wu, Lamiidae Reveal, Theidae Doweld - Asclepiadopsida Brongniart, Asteropsida Brongniart, Bignoniopsida Nees, Campanulopsida Bartling, Caprifoliopsida Endlicher, Coffeopsida Brongniart, Convolvulopsida Brongniart, Diospyropsida Brongniart, Ericopsida Bartling, Ligustropsida Meisner, Loniceropsida Brongniart, Myrsinopsida Bartling, Plantaginopsida Meisner, Primulopsida Brongniart, Rubiopsida Bartling, Selaginopsida Brongniart, Solanopsida Brongniart, Styracopsida Bartling, Verbenopsida Brongniart
[CORNALES + ×ERICALES] / Ericornids: ?
Age. C. Zhang et al. (2020) estimated that the age of this clade was around 120.1 Ma and X. Guo et al. (2021) suggested an age of 113.2 Ma.
Evolution: Genes & Genomes. There is a whole genome duplication, the COFLα event dated to ca 105.7 Ma, somewhere around here (Landis et al. 2018; see also Larson et al. 2019; O.T.P.T.I. 2019).
CORNALES Dumortier [parent of Ericales] / asterid IV of some studies - Main Tree.
Route I (route 2) secoiridoids, ellagic acid +, flavones 0; vessel elements with scalariform perforation plates; nodes 3:3; inflorescence cymose; K "small", C valvate, apparently free, (tube +, formation early); A basifixed; G inferior, with disc-like nectary, ventral carpellary bundles in the carpel wall [= transseptal bundles, i.e. vascular bundles to ovules go over the top of the septum and then down; no bundles run up the central axis of the ovary]; K persistent; germination epigeal, phanerocotylar. - 6 families, 51 genera, 590 species.
Includes Cornaceae, Curtisiaceae, Grubbiaceae, Hydrangeaceae, Hydrostachyaceae, Loasaceae, Nyssaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. Note that the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) suggest figures of (106-)101, 94(-89) Ma for the age of crown group Cornales and Anderson et al. (2005) similar ages of 101-97 My; Janssens et al. (2009) date the group to (117.1-)104(-90.9) Ma and K. Bremer et al. (2004a) to some 112 Ma, 115.3-105.1 Ma in Schenk and Hufford (2010: note topology) basically includes both. Magallón and Castillo (2009) estimate 101.55 Ma as its age, Lemaire et al. (2011b) (123-)106(-92) Ma, while ca 105.6 Ma was estimated by Fu et al. (2019a), around 104.1 Ma is the age in Magallón et al. (2015: note topology) and (117-)102(-90) Ma in Wikström et al. (2015). Some time in the Turonian, i.e. older than 89.8 Ma, perhaps as much as 93.9 Ma, is the estimate in Atkinson et al. (2018: Fig. 3), while at (95.6-)90.7(-86.9) Ma, the estimate in Tank and Olmstead (pers. comm.) is similar; see also the estimate of 114.1 Ma in C. Zhang et al. (2020: Table S5).
Fruits readily assignable to Cornales because of their distinctive anatomy are very well represented in the fossil record (Manchester et al. 2007, 2015). They can be dated to the Cretaceous-Maastrichtian, ca 70 Ma (Nyssa), Lower Campanian ca 82 Ma (Atkinson 2015), ca 80 Ma (Atkinson 2016: inc. Suciacarpa - septal bundles), some 84 Ma (Stockey et al. 2016a: Eydeia - septal vascular bundles), Coniacian, ca 87 Ma (Takahashi et al. 2002: Hironoia fusiformis - bundles in central axis), and ca 89 Ma from North America, material from three genera, with characters like germination flaps, rows of vascular bundles in the septae but no central bundles, and endocarp with sclereids or fibres on the inside (Atkinson et al. 2017b). Atkinson et al. (2017a) also described fossil fruits from the Upper Campanian on Vancouver Island ca 74 Ma old; see also Martínez-Millán (2010), Atkinson et al. (2016), also Nyssaceae below. Hill (1933) early discussed germination in Cornaceae s.l. in the context of fossils assignable to the family, emphasizing the behaviour of the germination flaps. Finally, the Mexican Operculifructus was found in Upper Cretaceous rocks ca 75 My; initially placed in Alismatales (Estrada-Ruiz & Cevallos-Ferriz 2007), it is now thought to be sister to Grubbiaceae, being linked by such features as a funnel-shaped endocarp, fused fruits, and absence of germination valves, if details of relationships in Cornales varied according to analysis (Hayes et al. 2018).
As to fossil flowers, the Late Cretaceous Silvianthemum suecicum, from rocks in southern Sweden ca 83.5 Ma, has tricolp(or)ate pollen, eight stamens (but a 5-merous perianth), the anthers appear to be dorsifixed, and there are three short, adaxially grooved styles (Friis 1990; see also Martínez-Millán 2010; Friis et al. 2011, 2013b). Bertilanthus scanicus, from the same rocks, has glandular hairs and stamens opposite the petals (Friis & Pedersen 2012). Although initially thought to be close to Quintinia (Paracryphiales), a position within Cornales was suggested by Beaulieu et al. (2013), which is more in accord with the morphology (and geography) of these fossils; Friis et al. (2013b) opt for a close linkage between Quintinia and the fossils, but they think that the phylogenetic position of Quintinia/Paracryphiales may be incorrect. Scandianthus, also Late Cretaceous and previously associated with Vahlia, may also belong in this area, indeed, there is quite a diversity of such 'saxifragalean' flowers in the fossil record (Friis et al. 2011: p. 489). Neither of the fossils mentioned was included in the study of Cornalean fossils by Atkinson (2018).
Evolution: Divergence & Distribution. In general, family identification of fossils is unclear, and they tend to have a mixture of characters of Cornaceae s.l. and Nyssaceae s.l.. In the comprehensive morphological analysis of Atkinson (2018) a number of fossils were associated with stem or crown Nyssaceae, while some were stem [Cornaceae + Nyssaceae], but in neither case was there much support. It is noteworthy that the morphospace occupied by these fossils overlapped only partly with that occupied by extant members of the order (Atkinson 2018). Genera like Hironoia fusiformis, from Cretaceous-early Coniacian deposists in Japan ca 89 Ma, may be best placed somewhere around Nyssaceae (Takahashi et al. 2002; see also Manchester et al. 2015; c.f. Yao et al. 2020: Aquifoliaceae), while Eydeia hokkaidoensis, some 84 Ma, has i.a. the distinctive septal vasculature of Davidia (Stockey et al. 2016a). Atkinson (2015) placed an 82 Ma fossil in this area although, as he noted, it has a novel combination of characters - the fruits are very large and the seed is described as being orthotropous, while Nguyen and Atkinson (2024) found 82-80 Ma fossils from deposits in Washington State, U.S.A. - members of another new genus, Fenestracarpa - which they placed in Cornales. Interestingly, in a joint analysis using 71 morphological characters from 38 fossil taxa and 48 extant taxa and 1 nuclear and 2 chloroplast markers from these extant taxa they found a family-level clade made up entirely of fossils - 9 genera, including the three just mentioned, and two unnamed fossils - that was sister to the [Curtisiaceae + Grubbiaceae] clade. The fossil clade was not named, although its suggested apomorphies are endocarps with an acuminate apex, elongated sclereids in the central axis and septa, and vascular bundles in longitudinal rows in the septa (Nguyen & Atkinson 2024: p. 8, Table 2).
With the exception of the separation of Grubbiaceae and Curtisiaceae, the clades recognised as families below all diverged within 10 Ma of the beginning of diversification of the order (Fu et al. 2019a).
It is difficult to optimise characters like stamen number on the tree given the extensive variation in the order. Endress (2011a) thought that the inferior ovary of Cornales might be a "key innovation" , although the clade is not very speciose, if morphologically quite diverse. For pollen evolution, see Y. Yu et al. (2018).
Genes & Genomes. There is little structural variation in the chloroplast genome in Cornales (Fu et al. 2017).
Chemistry, Morphology, etc.. The strands of apotracheal parenchyma are relatively long (at least 9 cells long) in Cornaceae s.l. (inc. Curtisiaceae) when compared with some of their putative relatives (Noshiro & Baas 1998). Spirally-thickened vessels holding the two halves of transversely-torn leaves together are quite common. Leaf teeth of Nyssaceae and Hydrangeaceae have a clear apex with a foramen, higher order laterals are involved (Hickey & Wolfe 1975).
The petals are often free, or appear to be free; corolla tube formation, when known, is early (e.g. Reidt & Leins 1994; Erbar & Leins 2011). Atkinson et al. (2016) described Cretaceous endocarps assignable to Cornales, they might have longituinally or tranversely elongated fibres, or no fibres at all, or both (Atkinson 2016), and the outer surface of the fruit might be ridged or smooth. There is considerable variation in seed size in this clade (Moles et al. 2005a), but seed size is not incorporated into the family characterisations.
For more details, see Faure (1924: general), Grayer et al. (1999: saponins) and Gousiadou et al. (2016: iridoids), Jahnke (1986: inflorescence), Ferguson (1977: pollen), Sato (1976: embryology), and Takahashi et al. (2002: fruits), mostly as Cornaceae s.l..
Phylogeny. Molecular studies (e.g. Xiang et al. 1993) early suggested a break-up of the old, broadly circumscribed Cornaceae; the core remains here. However, relationships between genera in this core remained unclear for some time, but at least some aggregation of the families they represent was clearly in order (e.g. Albach et al. 2001b; Xiang et al. 2002). Although Cornus is sister to Mastixiaceae in some morphological trees (Murrell 1993), it is not nearly so close in rbcL trees (Xiang et al. 1993, 1997). For the relationships of Grubbiaceae and Hydrostachyaceae (placement of the latter is particularly difficult, as we have seen), see especially Hempel et al. (1995), Xiang (1999), Soltis et al. (1997, 2000, 2007a), Savolainen et al. (2000b), Fan and Xiang (2003) and Xiang et al. (2002). In the analysis of nuclear genomes by C. Zhang et al. (2020), the relationships - all well supported - [Cornaceae [Curtisiaceae + Nyssaceae]] [Hydrangeaceae + Loasaceae]] were obtained; neither Grubbiaceae nor Hydrostachyaceae were included. There has been support for a sister group relationship between Grubbiaceae and Curtisiaceae for quite some time (e.g. Fan & Xiang 2001; H.-T. Li et al. 2021!).
Xiang et al. (2011) found a set of relationships [[Cornaceae [Curtisiaceae + Grubbiaceae]], Nyssaceae s.l., [Hydrostachyaceae [Hydrangeaceae + Loasaceae]]]]. Here taxon sampling is good and many of the relationships are well supported, however, nuclear 26S rDNA data alone suggested somewhat different relationships than did the six chloroplast genes, with Hydrostachys being embedded in Cornaceae s.l.. Using chloroplast genes alone a position of Nyssaceae as sister to [Hydrostachyaceae [Hydrangeaceae + Loasaceae]] had good support, and relationships suggested by this chloroplast phylogeny are followed here (see also most relationships in Schenk & Hufford 2010, but some nodes with weak support). A study using whole chloroplast genomes, but with skimpy sampling (15 genomes, four families), recovered similar relationships (Fu et al. 2017) as did the study by H.-T. Li et al. (2019); Fu et al. (2019a) added another 67 plastid genomes with the same result (see also below). However, there is little in the way of morphology to pin to the basal nodes, and as Xiang et al. (2011) noted, this new topology makes character evolution interesting. Morphological analyses (fruit characters) of extant plus fossil members of Cornales found quite strong support (93% bootstrap) for a [Cornaceae, Nyssaceae, Curtisiaceae, Grubbiaceae] clade (Atkinson 2018), but not for much else, while analyses that included only extant taxa showed very little support for any relationships.
S. K. Thomas et al. (2021) looked at relationships within Cornales using an Angiosperms353 probe set for 94 species; some >87% or more of the genes were recovered for all families except Hydrostachyaceae, where the figure was only 42%. Support was strong for the relationships [[[Cornaceae + Alangiaceae] [[Grubbiaceae + Curtisiaceae] [Mastixiaceae [Davidiaceae + Nyssaceae]]]] [Hydrostachyaceae [Hydrangeaceae + Loasaceae]]]. The position of Hydrostachyaceae was least supported, ancient reticulation, gene flow, and/or ILS perhaps being involved, but even so there was over 50% quartet frequency support for the main hypothesis (Thomas et al. 2021). Nguyen and Atkinson (2024) found a clade [Hydrostachaceae + Hydrangeaceae], but capsular-fruited members of Cornales were not the focus of their work. Although the topology [Hydrostachaceae [Hydrangeaceae + Loasaceae]] is that followed below, as already mentioned, a position as sister to all other asterids has also recently been suggested (W. J. Baker et al. 2021a), and clearly we do not really understand the relationships of this family, see above.
Classification. 11/15 of the genera of Cornaceae s.l. have been placed in monotypic families, or the family has been circumscribed very broadly, as by Mabberley (1997). How much the families on the Cornaceae s.l. are split still varies. Thus S. K. Thomas et al. (2021) recognized three more families than are recognized here, but the relationships between the taxa involved are the same, while Du et al. (2022) prefered to keep Cornus and Alangium in separate families - apparently because there were differences between the genera, some of the authors of the paper had sometimes argued for this circumscription before (however, others had adopted a broader circumscription) and the split between the two was relatively old. Ah, well.
Previous Relationships. The inhabitants of the old Cornaceae may be found in this site in Garryales (Garryaceae), Solanales (Montiniaceae), Asterales (Argophyllaceae) and Apiales (Griseliniaceae); Rodríguez C. (1971) grappled with possible relationships of Apiaceae, Araliaceae, etc., which centred on a "Cornalean alliance" - which at times has seemed to include taxa in just about all major pentapetalan group.
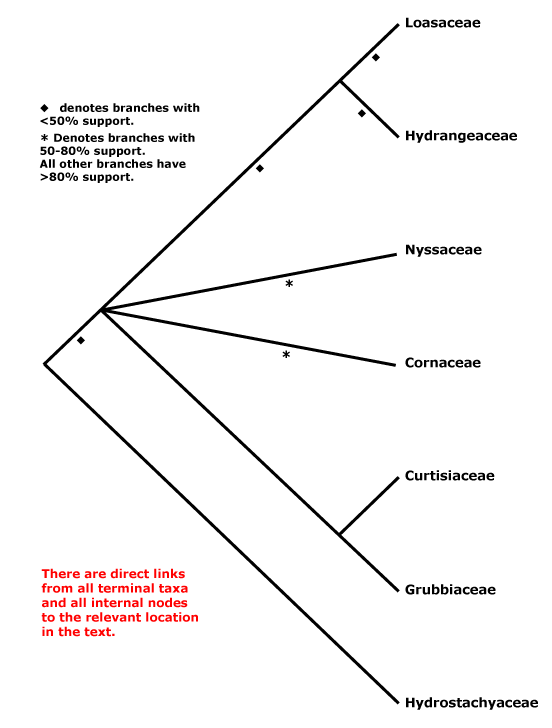
Synonymy: Alangiales Martius, Grubbiales Doweld, Hortensiales J. Presl, Hydrangeales Martius, Hydrostachyales Reveal, Loasales Berchtold & J. Presl, Nyssales Martius, Philadelphales Link
[Cornaceae [Nyssaceae [Grubbiaceae + Curtisiaceae]]]: sieve tube plastids also with polygonal protein crystalloids; leaves opposite, bases joined by a line/ridge; flowers small, 4-merous; G with symplicate zone 0; ovule 1/carpel, apical; fruit drupaceous, vascular bundles in central axis 0, K/T not persistent, drupe longitudinally grooved, walls with nests of sclereidal cells, endocarp sclereidal, thick, woody; seed coat smooth.
Age. This node may be around 97-95.5 Ma (Tank et al. 2015: Table S1, S2), 102.4-82.9 Ma (Schenk & Hufford 2010), ca 104.7 Ma (Fu et al. 2019a) or (113.2-)99.8(-85.1) (morphology) / (105.0-)104.1(-102.1) Ma (Du et al. 2022).
Evolution: Divergence & Distribution. Nguyen and Atkinson (2024) in their detailed discussion of fruit morphology and anatomy suggest a number of apomorphies for clades here; whether they will "stick" depends on the final topology.
CORNACEAE Berchtold & J. Presl, nom. cons. - Back to Cornales —— Synonymy: Alangiaceae Candolle, nom. cons.
Trees and shrubs (stoloniferous subshrubs), (deciduous); (thorns + - Alangium sect. A.); (plants Al accumulators), also route II decarboxylated iridoids, isoquinoline alkaloids, triterpenoid saponins, flavonols, +, (fructan sugars accumulated as isokestose oligosaccharides [inulins] - some Cornus), tanniniferous; (mucilage +); (laticifers +); (vessel elements with simple perforation plates); sclereids +; petiole bundle(s) arcuate (with adaxial inverted plate (and with wing bundles)), or D-shaped or annular (with inverted medullary bundle); hairs T-shaped (straight), unicellular, (stellate), walls often with crystals; (leaves spiral or two-ranked), lamina vernation conduplicate(-flat) or curved (both -plicate) or involute, margins entire/palmately lobed, secondary veins palmate, pinnate or actinodromous; (plant dioecious); (inflorescence capitate); flowers (-10 - A.)-merous; K notably small, connate or not, (decussate), C (decussate); A = and opposite K (-4 x A, anthers long - A.); pollen with complex endaperture [a pore joining two lateral thinnings parallel to the colpus], often starchy, exine with granules or spinules [C.], to 8-aperturate, lumina + muri [striate, reticulate, rugulate] or columellate-capitate [A.]; G 1-2(-3)-locular, style short/long, stigma truncate, ± capitate (with 4 longitudinal bands), (bilobed), dry; ovules apotropous, raphe dorsal/lateral, parietal tissue ca 3 cells across (0 - red-fruited C.), hypostase 0; (megaspore mother cells several), (embryo sac tetrasporic, 8-nucleate, antipodals polyploid [Fritillaria type]); fruit a drupe, 1-2-seeded, wall sclereidal, (with cavities - subgenus C.), septum with (elongated and) isodiametric sclereids, germination valve elongate [most of length of stone]; testa of elongated cells, much compressed, (ca 6 cells thick, vascularized - A.); endosperm (also nuclear), hemicellulosic, embryo chlorophyllous; n = 8-11, x = 6 (?7), nuclear genome [1 C] (0.087-)1.662(-31.777) pg; (plastid transmission biparental).
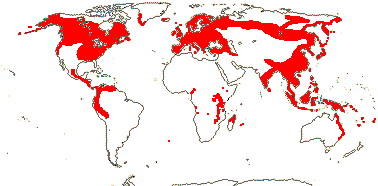
2/115: [list]. Cornus (65), Alangium (50). Scattered, not S. South America. Map: see van Steenis and van Balgooy (1966), Aubréville (1974), Fl. Austral. vol. 8 (1984), Meusel et al. (1978), Hultén and Fries (1986), Xiang and Thomas (2008) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 6. (2011). Photos - Habit, [Photo: Cornus Inflorescence, Flower, Fruit.
Age. An age of (82-)67(-47) Ma is suggested for crown-group Cornaceae (Bell et al. 2010); Wikström et al. (2001) suggested an age of (79-)73, 64(-58) Ma, Fu et al. (2019a) an age of ca 93.3 Ma while (107.1-)92.6(-77.4) (morphology) / (104.9-)103.2(-100.2) Ma were the estimates in Du et al. (2022).
Evolution: Divergence & Distribution. Xiang and Thomas (2008) thought that stem-group Cornus was Late Cretaceous in age, ca 80 Ma old, substantial diversification having occurred by ca 66 Ma. Fruits of Cornus subg. Cornus have distinctive alveolate endocarps and are known from the Palaeocene of North Dakota in rocks about 58 Ma old; they have six locules (Manchester et al. 2010b). Manchester et al. (2015) also discuss the fossil record, and they note that pollen of Alangium can be confused with that of Pelliciera (Ericales), while fossil wood of Alangium is reported from Eocene deposits in Oregon, interesting distributionally (Wheeler & Manchester 2002).
For a study reconstructing ancestral areas and characters in Cornus, see Xiang and Thomas (2008); the results depended much on the methods used, etc.. Cornus oblonga, from eastern Asia, is sister to C. peruviana, known from Costa Rica to Bolivia (Xiang et al. 2006). Du et al. (2022: q.v. for numerous dates, etc., sampling good) developed this further, carefully integrating fossils, etc.. They suggested the genus originated in E. Asia, with its current subglobal distribution being explicable by a scenario that involved some 20 dispersal and 15 vicariance events. Cornus is an old clade, and the four main clades within it diverged within some 7 My at about the end of the Cretaceous; C. parviflora is sister to C. peruviana, but the geography is the same (C. parviflora is also E. Asian), except it all happened around 20 My later (Du et al. 2022)...
Pollination Biology. Some of the floral organ B (i.e. not PI, but AP3) and C genes are expressed in the large, white inflorescence bracts of the pseudanthia of species like Cornus florida, an heterotopic shift that has occurred at least twice. However, such bracts are not morphologically equivalent in other species, e.g. in C. canadensis (Maturen et al. 2005; Xiang et al. 2010; W.-H. Zhang et al. 2008: extensive PI-like gene duplication; Feng et al. 2012 - see also Nyssaceae below). For inflorescence architecture in Cornus, in particular for the role of CorLFY genes there, see J. Liu et al. (2013), while Ma et al. (2017a) looked at the effect of the amount and timing of expression of CorTRL1 and CorAP1 on inflorescence architecture.
The anthers of Cornus canadensis have explosive dehiscence based on the trebouchet principle. Anthers dehisce in late bud but the stamens are held under tension by the connivent apices of the petals. Perhaps triggered by an insect touching the awns that are found on one or two of those petals (when I first saw them, I had no idea what they might do), the filaments straighten rapidly and the pollen is flung upwards (Whitaker et al. 2007: ?C. suecica). The maximum acceleration rate of the pollen grains has been estimated at 24,000 m/s2 (Edwards et al. 2011). Despite the flower-like appearance of the inflorescence, wind pollination may also occur (Whitaker et al. 2007).
Chemistry, Morphology, etc.. Blue-fruited dogwoods have lost iridoids (Xiang et al. 1997). In nodes of Alangium the central vascular trace may immediately divide into three (nodes 3:5). Some species of Alangium have sympodial growth (de Wilde & Duyfjes 2020), as does Cornus kousa, etc.. Mabberley (1997) describes Alangiaceae as having spiral leaves; they are often two-ranked.
Alangium has very little vascular tissue in the center of the ovary, although it does have septal vascular bundles. There is considerable variation in embryo sac development in Cornus in particular (Johri et al. 1992 for references).
For further information, see Kubitzki (2004b) and de Wilde and Duyfjes (2020: Alangium), both general, Jensen et al. (1975a: iridoids), Adams (1949: anatomy), Mittal (1961) and Neubauer (1978), both petiole anatomy, Feng et al. (2011: inflorescence morphology and evolution), Reidt and Leins (1994: corolla of Alangium), Eyde (e.g. 1968, 1988: flower and fruit in particular), Reitsma (1970) and Ferguson (1977), both pollen, Fagerlind (1939c: embryo sac), and Manchester et al. (2010b) and Morozowska et al. (2021), both fruit morphology/anatomy.
Phylogeny. Extensive work has been carried out on relationships within Cornus, e.g. Murrell (1993) and Xiang et al. (1993, 2006), Xiang and Thomas (2008) and Feng et al. (2011). Relationships in S. K. Thomas et al. (2021) were [dwarf dogwoods [blue- and white-fruited dogwoods [cornelian cherries + big-bracted dogwoods]]]. Du et al. (2022) carried out a variety of analyses; the Angiosperms353 analysis used 56 samples and 312 genes, a plastome analysis, 21 samples, 79 protein-coding genes, 48 samples and 24,690 loci in a RADSeq analysis, and so on. The basic groups they recovered, [BW [CC [DD + BB]]], had been obtained in some previous analyses, but were somewhat different from those in Thomas et al. (2021: [DW [BW [CC + BB]]]), although they found that some limitations in the analyses carried out by the latter explained the differences. For relationships within Alangium, see e.g. Feng et al. (2009).
Classification. For a discussion of infrageneric classifications of Cornus, 10 subgenera being recognized, see Xiang et al. (2006). There are major clades in the genus, so subgenera (for those clades) and sections might be the way to go. Du et al. (2022) follow Phylocode principles in their naming of 12 clades within Cornus - note that Cornus is a node‐based name and the Cornaceae that thet talk about include only Cornus and its stem, i.e. not Alangium, so it is not the same as Cornaceae above, alack, or in A.P.G. IV (2016).
[Nyssaceae [Grubbiaceae + Curtisiaceae]]: ?
NYSSACEAE Dumortier, nom. cons. - Back to Cornales, —— Synonymy: Camptothecaceae Chen, Davidiaceae H.-L. Li, Mastixiaceae Calestani
Trees and shrubs; (Al accumulators); triterpenoid saponins, (resin), +, tanniniferous, (mucilage +); (laticifers +); (leaf traces running along the stem - Mastixia), (sclereids +), pith septate or not; petiole bundles arcuate or with adaxial plate; (stomata paracytic); hairs T-shaped, unicellular; leaves spiral (opposite), lamina vernation conduplicate [Nyssa, N.], margins serrate or entire; plants andromonoecious, dioecious, etc., or flowers perfect; inflorescences various, racemose, (capitate); flowers usu. (4-)5-merous; P 0, or K notably small, C ± imbricate, or valvate and inflexed at apex and with an adaxial median ridge; A 4-26 [often diplostemonous]; pollen with complex endaperture [a pore joining two lateral thinnings parallel to the colpus], tectum perforate [?all]; (nectary 0 - Davidia); G [1(-3), (6-10)-locular], style short, (long - N.; branched); ovules epitropous, micropyle long, integument ca 8-10(-15+) cells across, parietal tissue 1-3 cells across, (nucellar cap ca 2 cells across), suprachalazal zone much elongated, hypostase +, supraraphal chalaza massive, (pachychalazal), (endothelium +); (megaspore mother cells several); fruits 1-5-seeded, drupe walls with intertwining fibres; germination valve short, at apex of loculus, or elongate, septum with fibres (sclereids), (vasculature as longitudinal rows - Davidia), septal ridge on stone [?all], (seed U-shaped in t.s. because of intrusive germination valve - Mastixia); testa multiplicative, exotesta lignified, cells polygonal; (endosperm also nuclear), embryo long or short; n = 11, 13 [both N.], 21, 22.
check: sieve tube plastids also with polygonal protein crystalloids; drupe longitudinally grooved, walls with nests of sclereidal cells.
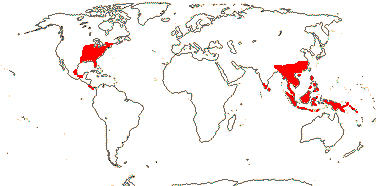
5/32: [list], Mastixia (19). Mainly East Asia, also Indo-Malesia and E. North America. Map: see van Steenis and van Balgooy (1966) and Matthew (1977). [Photo - Nyssa Flower, Fruit © H. Wilson.]
Evolution: Divergence & Distribution. Manchester et al. (2009 and references, 2015) discuss the early Caenozoic fossil history of what are now East Asian endemic genera of Nyssaceae. Fossils of mastixioid fruits in particular are widespread in the northern hemisphere in the Late Cretaceous and Caenozoic-Palaeogene, some being 3- or 4-carpelate; they are placed in 8 genera and 33 species (Eyde & Qiuyun 1990; Eyde 1997; Manchester & Collinson 2022 for details: 8 genera from the London Clay flora alone). Mastixia, now India to China to East Malesia, was especially abundant in Europe and Siberia 65-70 Ma (Manchester 2002), and pollen is known from Austria in deposits only some 17-11 Ma, and pollen like that of the North American Nyssa sylvatica is also to be found there (Hoffmann & Lichtenwagner 2019). See also Kvacek (2008).
A number of fossils are placed as crown- or stem-group Nyssaceae by Atkinson (2018), although Hironoia is stem [Curtisiaceae [Nyssaceae + Cornaceae]] and placed sister to Amersinia, also initially thought to be close to Nyssaceae (Manchester et al. 1999), albeit with little support.W. Zhou et al. (2020) discuss the biogeography of Nyssa; it shows extensive movements around the Northern Hemisphere, rather like Cornus, which is similar in age (crown-group Nyssa ca 57.7 Ma, with Davidia ca 72 Ma).
Pollination Biology. Davidia has numerous perianth-less flowers in capitulae that are subtended by 2 large white bracts (see Baczynski & Claßen-Bockhoff 2023 for these pseudanthia). Expression of B-class floral genes in these bracts is implicated (Vekemans et al. 2011). Indeed, it is very difficult to recognize staminate flowers, the inflorescence meristem having many features of a floral meristem, e.g. lacking acropetal growth, and hence it has been called a floral unit meristem - see also Asteraceae (Claßen-Bockhoff & Arndt 2018).
Plant-Animal Interactions. For the indole alkaloid camptothecin, found in Camptotheca acuminata (and also in some Rubiaceae, Apocynaceae and Olacaceae) see Lorence and Nessler (2004). Although the enzyme that camptothecin targets in the herbivore - DNA topoisomerase I, with the resultant induction of cell death - is also found in the plant, it is probably protected by changes in its amino acid sequence, one of which (serine in position 722) is the same as is found in camptothecin-resistant tumours in humans (Sirikantaramas et al. 2009). Camptothecin is sequestered in glandular hairs, although not in the laticifers (Hagel et al. 2008).
Genes & Genomes. For some cytology, see Z.-C. He et al. (2004).
Chemistry, Morphology, etc.. Diplopanax contains petroselenic acid (Zhu et al. 1998). Although Zhu et al. did not find petroselenic acid in Fatsia (Araliaceae) or Aucuba (Garryaceae), it is found in a number of Apiales, and relationships between Cornaceae and some Apiales have been suggested in the past.
Mohana Rao (1973a) described the integument of Nyssa as being ca 5 cells across and the ovules as being tenuinucellate. However, the integument is much thicker when the embryo sac is mature (ibid: Fig. 5G), and a single subepidermal layer of cells is shown between the embryo sac and epidermis (ibid.: Fig 3D, E); epidermal cells on occasion divide periclinally, but such divisions seem not to account for this subepidermal layer.
Davidia lacks a perianth and may have bitegmic ovules (but c.f. Horne 1914). Diplopanax has recently been placed in Mastixiaceae s. str. (Eyde & Xiang 1990; c.f. Xiang et al. 1997). It has five lobes on the disc opposite the corolla and a single-seeded fruit the embryo of which is C-shaped in transverse section (Ying et al. 1993); see above for chemistry.
For general information, see Kubitzki (2004), for the chemistry of Camptotheca, see Chang et al. (2020), for floral development, see Gong et al. 2018 (Camptotheca, also family summary), for pollen, see Ferguson (1977: Mastixia), and for some embryology, see also Horne (1909, 1914), Tandon and Herr (1971). For Mastixia, see Matthew (1976); embryological details are unknown for it, Diplopanax, etc.
Phylogeny. Z.-D. Chen et al. (2016) obtained the relationships [Diplopanax + Mastixia] [Davidia [Camptotheca + Nyssa]]], with good support. Relationships obtained by S. K. Thomas et al. (2021) are similar, although three families, [Mastixiaceae [Davidiaceae + Nyssaceae]], were recognized there.
[Grubbiaceae + Curtisiaceae]: style short, lobed; ovules epitropous, parietal tissue 0; vascular bundles in central axis +; endosperm copious.
Age. The age of this clade is ca 90 Ma (Warren & Hawkins 2006); ca 59.4 Ma is the age in Magallón et al. (2015) and ca 82.5 Ma in Fu et al. (2019a).
Evolution: Divergence & Distribution. Members of this clade are now restricted to the Cape in South Africa, and the ages above might suggest that they are very much relicts in the Cape flora (e.g. Warren & Hawkins 2006), however, distributions of fossils suggest a more complex history. Thus Manchester et al. (2007a) found fossils that were associated with Curtisiaceae in Early Eocene deposits in England, while Hayes et al. (2018) linked fossils of Operculicarya from the Upper Cretaceous of Mexico with Grubbiaceae.
Chemistry, Morphology, etc.. For characters holding these two families together, see in part Xiang et al. (2002).
Classification. Xiang et al. (2002) suggested that Grubbiaceae and Curtisiaceae might be combined, but they are kept separate here because they are rather different in appearance (see also A.P.G. III 2009).
GRUBBIACEAE Meisner, nom. cons. - Grubbia Bergius - Back to Cornales —— Synonymy: Ophiraceae Arnott
Evergreen ericoid shrubs; iridoids 0?; sieve tube plastids?; subepidermal collenchyma +; hairs unicellular; cuticle waxes as long narrow plateles; leaves ± ericoid [lamina margins revolute]; inflorescences axillary, capitate or cone-like; flowers also 6-merous; P [= C?] valvate; A 8 (12), longer opposite P, bisporangiate, dithecal, extrorse; pollen surface ± smooth, ?endapertures; G [2], transverse, placentation axile at base, becoming free-central; nectary hairy; ovule integument "thick" [4-6 cells?], micropyle "long", hypostase +; fruit a syncarp; ?endocarp, stone ?grooving; 1 seed/syncarp [?always]; exotestal cells ± boat-shaped in t.s., tanniniferous, other cells ± collapsed; micropylar and chalazal endosperm haustoria +, embryo long; n = ?
1/3: [list]. Cape Floristic Province, South Africa. Map: from Vester (1940).
Chemistry, Morphology, etc.. The inversion of the anther is supposed to be very comprehensive in Grubbiaceae, and for some (e.g. Fagerlind 1947b) this suggested relationships with Ericaceae. Similarly, Cronquist (1981: pp. 472, 473) noted that that the anthers "minute, dithecal, inverted" appeared to be extrorse, all in all, the stamens were "very suggestive of the Ericales" (= Ericaceae and immediate relatives), which is where he put the family. The filaments seem strongly incurved in the recently-opened flower, and then they straighten; Kubitzki (2004b) was unclear as to what exactly was going on. The vascularization of the seed is unclear, vascular bundles reported as being found in "certain parts of the seed coat" (Yembaturova et al. 2009: p. 90).
Some information is taken from Carlquist (1977), Dahlgren in Dahlgren and van Wyk (1988) and Kubitzki (2004b), all general, Carlquist (1977b: wood anatomy, 1978a: vegetative anatomy), Schnizlein (1843-1870: fam. 18, carpel orientation), and Fagerlind (1948b: embryology).
The family is poorly known.
Previous Relationships. Carlquist (1977b, 1978a) found Grubbiaceae to be anatomically identical to Bruniaceae (Bruniales, a campanulid) and also very similar to Geissolomataceae (rosid-Crossosomatales), being generalized "rosoid", he thought, in anatomy; Thorne (1968) also included families now placed in Gunnerales and Ericales that he thought were related, and relationships with Santalales, etc., have also been suggested in the past....
CURTISIACEAE Takhtajan - Curtisia dentata (N. L. Burman) C. A. Smith - Back to Cornales
Evergreen trees; ?ellagic acid; ?nodes; petiole bundle annular, with medullary strands; single crystals +; lamina vernation ± flat, margins serrate; inflorescence terminal; K small, open; A = and opposite K; pollen with complex endaperture [a pore joining two lateral thinnings parallel to the colpus]; G [2-4], with axial/central vascular bundles; fruit usu. 4-seeded, mesocarp with sclereids, endocarp of large sclereidal cells with horizontally-elongated digitate-interlocking walls, germination valve elongated, septum with isodiametric sclereids; endotesta tanniniferous, rest ± collapsed; ?endosperm haustoria, embryo minute; n = 13, x = 12 (?11).
1/1: [list]. Southern Africa. Map: from Coates Palgrave (2002) and Yembaturova et al. (2009); fossil [blue] from Manchester et al. (2007a). Photo - Fruit.
Age. Manchester et al. (2007a) recognised the distinctive fruits of Curtisia from the early Eocene London Clay of ca 55 Ma; the fossils were originally placed in Epacridaceae (= Ericaceae-Styphelioideae).
Evolution: Divergence & Distribution. Curtisia is known fossil from southern England (Manchester et al. 2007a, 2015); see also Ericales-Roridulaceae for a comparable extant/fossil distribution.
Chemistry, Morphology, etc.. Takhtajan (1997) described the hairs of the branchlets, petioles and inflorescences of Curtisia as being stellate; they are simple and curled. The "plications" (Cullen 1978) in the young leaves are in fact only prominent veins.
Curtisia is embryologically unknown, but it lacks transseptal bundles, having the "normal" central bundles.
For general information, see Kubitzki (2004b), for pollen, see Ferguson (1977).
Classification. See Yembaturova et al. (2009).
[Hydrostachyaceae [Hydrangeaceae + Loasaceae]]: chlorogenic acid +; G with intrusive parietal placentation; ovules many/carpel, parietal tissue 0; fruit capsular, septicidal [so no germination valves, etc.]; micropylar endosperm haustorium +.
Age. This clade is ca 96.7 Ma (Fu et al. 2019a).
HYDROSTACHYACEAE Engler, nom. cons. - Hydrostachys Thouars - Back to Cornales
Annual to perennial herbs; submerged aquatics; primary root 0, "adventitious" roots +; kaempferol +, iridoids 0; vessel ?type; nodes ?; stomata 0; leaves spiral, deeply and complexly divided, surface with small enations, stipules +, single, intrapetiolar (two, lateral); inflorescences in groups, (axillary), densely spicate, plants di(mon)oecious; bracts persistent, usually ornamented axbaxially; bracteoles 0; P 0; two lateral tufts of multicellular ?uniseriate hairs; nectary 0; staminate flowers: A 1, "extrose"; pollen in tetrads, inaperturate; carpelate flowers: G superior, [2], transverse, style branches separate, filiform, impressed; ovules with integument ca 5 cells across, ?endothelium; seeds minute, exotestal, outer cell walls much thickened, mucilaginous; endosperm scanty or 0; n = 10-12, x = 6 (?12, ?7).
1/20: [list]. C. and S. Africa, Madagascar. Map: from Rauh and Jäger-Zürn (1966b) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
Chemistry, Morphology, etc.. The caffeoyl ester chlorogenic acid is found here and in the Loasaceae-Hydrangeaceae clade (Rønsted et al. 2002). Vessels are reported (Jäger-Zürn 1998), but are not described. Cusset (1973) described and illustrated the leaves as having stipules, but what these structures "are" is unclear.
One interpretation of the androecium is that it consists of a single tetrasporangiate stamen (dehiscence extrorse - Cusset 1973) or that it consists of two bisporangiate anthers with more or less connate filaments. There seems not to be an endothelium. The styles are more or less impressed into the apex of the ovary, a feature that Leins and Erbar (1988) noted was common in Lamiales, although I do not know the general distribution of this feature.
For floral development, see Leins and Erbar (1988), for general information, see Erbar and Leins (2004a), and for chemistry, Rønsted et al. (2002).
Previous Relationships. Hydrostachyaceae have variously been suggested to be sister to Decumaria (Hydrangeaceae) (Albach et al. 2001), or close to Crassulaceae (Saxifragales), or - perhaps - close to Podostemaceae (sister to Hypericaceae, in Malpighiales). However, Takhtajan (1997) included Hydrostachyales in his Lamiidae, Cronquist (1981) also put Hydrostachyaceae in that general area, and Burleigh et al. (2009) also suggested they might be in Lamiales.
[Hydrangeaceae + Loasaceae]: similar route I secoiridoids and route II decarboxylated iridoids [C-8 iridoid glucosides +; C-9 iridoids, keeping the C-11, e.g. deutzioside, +], flavonols +, ellagic acid 0; cork cambium deep-seated; hairs tuberculate, walls calcified, with basal cell pedestals; leaves opposite, lamina (lobed), margins with glandular teeth; C clawed, often concave [= boat-shaped]; A (2x C-)many, (initiated as antesepalous triplets), centripetal; G semi-inferior, with axial/central vascular bundles, stigma dry; ovules with endothelium; fruit with (persistent placental strands +, valves apical; exotestal cells variously elongated, inner walls thickened; endosperm ?, chalazal endosperm haustorium +, embryo straight; mitochondrial coxII.i3 intron 0.
Age. A possible age for this node is (64-)50, 49(-36) Ma (Bell et al. 2010), ca 70.4 Ma (Tank et al. 2015: Table S2; ca 36.8 Ma Table S1), while Wikström et al. (2001) suggest an age of (96-)91, 82(-77) Ma, Schenk and Hufford (2010) an age of 91.6-58 Ma, while around 90.5 Ma is the age in Magallón et al. (2015) and ca 95.4 Ma that in Fu et al. (2019a).
Evolution: Divergence & Distribution. It is possible that diplostemony is plesiomorphic, with polystemony derived. The antisepalous androecial triplets sometimes found here are also found in Rosaceae and Zygophyllaceae (Hufford 2001b, see also Ronse Decraene & Smets 1996a)! The scabrid-glochidiate hairs that characterize Loasaceae are also found in Jamesia/Fendlera, sister to other Hydrangeaceae; they could be an apomorphy for the family pair, and then lost in most Hydrangeaceae... Weigend (2004) notes a number of features that may unite this family pair; not all have been incorporated above, and some have been placed at the [Hydrostachyaceae [Hydrangeaceae + Loasaceae]] node.
Chemistry, Morphology, etc.. For some distinctive iridoids found in this family pair, see Frederiksen et al. (1999) and Gousiadou et al. (2016).
The androecium of both families is very variable in development (Hufford 1990, 1998). The embryology of the group is poorly studied. Fruits are sometimes septicidal and sometimes loculicidal.
Classification. Takhtajan (1997) included Hydrangeales in Cornidae-Cornanae, but Loasales-Loasanae were part of his Lamiidae.
HYDRANGEACEAE Dumortier, nom. cons. - Back to Cornales
Shrubs, vines, or herbs; (plants Al accumulators); kaempferol, flavonols +, tanniniferous; cork inner cortical or outer pericyclic; (vessel elements with simple perforation plates); true tracheids +; (stomata paracytic); leaves opposite, bases joined by lines across the stem, lamina vernation conduplicate or supervolute, (secondary veins palmate); inflorescence cymose; flowers 4-5(-10)-merous; anthers with basal pit; tapetal cells 1-4-nucleate; nectary vascularized; G [(2-)3-5(-12)] to inferior, ovary ribbed, arrangement variable, stigma linear to capitate; ovules apotropous, integument (3-)5-7(-10 - Hydrangea) cells across; embryo sac protruding into micropyle, antipodal cells persist; seeds winged or not; endosperm moderate, micropylar endospermal cells elongated; n = 13-18, x = 12 (?9, ?13), nuclear genome [1 C] (0.191-)1.463(-11.191) pg.
9/270 (223): [list], to tribes. Warm temperate, some species in tropics. [Photo - Flower, Collection.
Age. The age for crown Hydrangeaceae may be (87-)83, 69(-65) Ma (Wikström et al. 2001) or (58-)44, 43(-30) Ma (Bell et al. 2010); both include Hydrostachyaceae. Ca 52 Ma is the age in C. Kim et al. (2015b)[and ca 45 Ma (C. Kim et al. 2015)?].
Some of the Late Cretaceous fossils assigned to Esgueiria and placed in Combretaceae may end up here - c.f. style, hair surface, etc. (see Takahashi et al. 1999). The Late Cretaceous Scandianthus from Sweden, has also been linked with Hydrangeaceae (see Friis & Pedersen 2012 for references). Tylerianthus crossmanensis, in Late Cretaceous deposits from eastern North America of ca 90 Ma age, has nectaries on the upper part of its inferior ovary, five sepals, and five stamens opposite the sepals that alternate with five long, linear staminodes. It has been often compared with Hydrangeaceae (see also Crepet et al. 2004), Saxifragaceae s. str. (including Parnassiaceae, Gandolfo et al. 1998b), Grossulariaceae (see Friis et al. 2014) and Saxifragales (López-Martínez et al. 2023a: Table 3), and in this latter case a position in Cornales was never an option. In the angiosperm-wide analysis recently carried out by Schönneberger et al. (2020) it ended up in crown-group Saxifragaceae, although there were a dozen other positions (unspecified) just one step longer.
1. Jamesioideae Hufford
Shrubs; (myricetin +), iridoids 0 [Jamesia]; hairs scabrid-glochidiate; leaf buttresses prominent after leaves fall; K valvate; A 10, filaments flattened, ± forming tube; G (3-)4-5, style branched (almost) to the base; endothelium 0, basal part of nucellus persists [Fendlera]; endosperm nuclear [Fendlera]; n = 16.
2/5: Fendlera (3). W. North America. Map: from Holmgren and Holmgren (1989).
Age. Crown-group Jamesioideae are around 25 Ma (C. Kim et al. 2015b).
2. Hydrangeoideae Burnett
Phloem loading via intermediary cells [specialized companion cells with numerous plasmodesmata, raffinose etc. involved]; nodes also 5:5, 7(+):7(+); (hairs stellate or branched); petiole bundles (arcuate [+ inverted bundles])/annular, often with medullary bundles; raphide sacs + (0); stomata variable; (hairs stellate); K valvate, C (contorted), connate [?level]; A (10/initiated as triplets opposite K); (placentation axile); (archesporial cells several), embryo sac long [inc. long chalazal half], ± grows out of micropyle; ?fruit.
15/185 - two tribes below. Warm temperate, esp. South East Asia and North America, S. to Chile and Malesia (map: from Hu 1955; Zaikonnikova 1966; McClintock 1957; van Balgooy 1984; Mai 1985; Hong 1993).
Age. The age of crown Hydrangeoideae is estimated at (82-)78, 67(-63) Ma (Wikström et al. 2001: note position of Hydrostachys!) or ca 43 Ma (C. Kim et al. 2015).
2A. Philadelpheae Duby —— Synonymy: Kirengeshomaceae Nakai, Philadelphaceae Martynov
Shrubs (perennial herbs); (stomata laterocytic); (K connate), C imbricate; A (10/many), initiated on five common primordia; style ± branched or not; ovule with 1 lateral layer of nucellar tissue.
6/130: Philadelphus (65 or fewer), Deutzia (60). Warm temperate, esp. South East Asia to the Philippines, S.W. North America, also Central America, Philadelphus coronarius in Europe.
Age. The age of the clade [Kirengeshoma + the rest] is 70.6-33.9 Ma (Guo et al. 2013).
2B. Hydrangeeae de Candolle - Hydrangea L. —— Synonymy: Hortensiaceae Martinov
Shrubs, (root climbers), deciduous or not; myricetin + [sect. Decumaria]; raphides +; inflorescence often with conspicuous marginal flowers [= large C-like K]; C valvate; A (5/many), anthers C-shaped, connective well developed; microsporogenesis simultaneous [Platycrater]; style +, short to long, ± separate; fruits loculicidal, endocarp of large cells with horizontally-elongated digitate-interlocking walls, (baccate).
1/65. Warm N. temperate, esp. East Asia, S. to Chile and Malesia, Hawaii.
Evolution: Divergence & Distribution. X. Yang et al. (2023) saw divergence in Hydrangea as being driven by changes in geology and climate, resource limitation, range fragmentation, and so on.
Pollination Biology & Seed Dispersal. Wong Sato and Kato (2019) found that the conspicuous sterile marginal flowers common in Hydrangea (≡ petals) tended to increase the frequency of visits by potential pollinators. Some species are noted for changing colour from pink or red to blue as soil acidity and the concentration of Al+++ ions increases.
Chemistry, Morphology, etc.. Species of Deutzia have stamens in a single whorl with strongly flattened filaments that may form a tube around the ovary and style (see also C. Kim et al. 2015), and a similar arrangement occurs elsewhere in the family (e.g. in Jamesia). Philadelphus has centrifugal androecial development, while in Platycrater arguta (= Hydrangea) the carpels are initiated well before the stamens (Ge et al. 2007); see also Remizowa (2019) for comments. In Philadelphus, Deutzia, and Hydrangea sect. Dichroa the four carpels alternate with the sepals, or there are three carpels with the odd member adaxial; in other species of Hydrangea the odd carpel is abaxial or there are five carpels opposite the sepals. There is considerable variation in the degree of fusion of the styles, but transitions between the extremes are easy to envision (Roels et al. 1997). In a number of taxa the embryo sac more or less protrudes into the micropyle or beyond (Maheshwari 1950; Hufford 2004). The presence of chalazal haustoria needs confirmation; Mauritzon (1933) noted that the antipodal cells might persist, as in Kirengeshoma, and talks of an "Antipodenhaustorium". The base of the endosperm is lignified; Fendlera has nuclear endosperm (Mauritzon 1939a). The endocarp of Hydrangea consists of large cells with horizontally digitate-interlocking anticlinal walls, also found in Curtisia, but not in Cornus (Manchester et al. 2007).
For general information, see Hufford (2004), for iridoids, see Frederiksen et al. (1999) and Gousiadou et al. (2016) and for flavonoids, see Bohm et al. (1985), for vegetative anatomy, see Watari (1939), Styer and Stern (1979 and references) and Gregory (1998), for variation in the position of the carpels when the gynoecium is bicarpelate, see Eichler (1878; also Eckert 1966), for floral anatomy and morphology, see Klopfer (1971, 1973) and Bensel and Palser (1975c), for androecial development, Gelius (1967) and Hufford (1998, 2001a), for floral morphology of Hydrangeae, see Hufford (2001) and Ge et al. (2003), for pollen, see M. Zhang et al. (2019: Hydrangea), for some embryology, etc., see Gaümann (1919: Philadelphus), Mauritzon (1933, 1939a) and Ao (2008), and for seeds, see Hufford (1995, 1997) and Nemirovich-Danchenko and Lobova (1998)
Phylogeny. For relationships within the family, see Hufford (1997b), Hufford et al. (2001: support for its monophyly is not overwhelming), Soltis et al. (1995a) and C. Kim et al. (2015b). Rleationships in C. Kim et al. (2015) are [Philadelphoideae [Jamesioideae + Hydrangeoideae]].
Z.-D. Chen et al. (2016) show relationships within Chinese Hydrangeoideae, quite diverse. Samain et al. (2010b) and Granados Mendoza et al. (2012) discuss relationships in Hydrangeeae, where Hydrangea is polyphyletic, and relationships there were further clarified by de Smet et al. (2015: quite good support for many deeper branches). Guo et al. (2013) studied relationships around Philadelphus, within which Carpenteria may be embedded. The couple of species of Deutzia from Mexico are sister to the rest of the genus (C. Kim et al. 2015b).
Classification. For a classification of Hydrangeaceae, see Hufford et al. (2001). Generic limits around Hydrangea were a mess - Platycrater and seven other genera are embedded in Hydrangea s. str. - but although the circumscription of Hydrangea has been extended and a sectional classification provided (de Smet et al. 2015), some prefer narrower generic limits (Ohba & Akiyama 2016).
Previous Relationships. Hydrangeaceae were part of the old woody Saxifragaceae/Saxifragales, or included with members of this group in Rosales (e.g. Cronquist 1981).
LOASACEAE Jussieu, nom. cons. - Back to Cornales
Often coarse herbs (shrubs); myricetin, tannins 0; cork inside pericycle; vessel elements with simple perforation plates; petiole bundles arcuate or annular, with wing bundles; indumentum complex, trichomes unicellular, glochidiate, walls also with calcium phosphate (0), silicification +/0; leaves opposite at least basally, otherwise spiral, (compound), lamina (margins lobed), secondary veins pinnate-palmate; inflorescences terminal, branches cymose; flowers (4-)5(-7)-merous; K connate, "large" [usu. enclosing bud], C with three traces; C-A synorganisation, C-A plate formed, A many, filaments terete, [staminodia 0]; pollen tectum striate; G [5], (± superior), opposite sepals, (3, odd member adaxial), style hollow, lobed, stigma narrow or clavate; ovules epitropous, integument 12-17 cells across; K usu. persistent in fruit; (testa with hypodermal layer thickened); endosperm copious to none [?distribution]; cotyledons apically emarginate, with apical hydathodal projection; x = 12 (?13, ?11).
21/350: [list], to tribes as far as possible - five clades below. Mostly American, but also Africa and the Marquesas Islands. Map: from Heywood (1978).
Age. Possible crown-group ages for Loasaceae are (44-)33, 31(-21) Ma (Bell et al. 2010) or (73-)67, 63(-57) Ma (Wikström et al. 2001).
1. Eucnideae Gilg - Eucnide Zuccarini
Herbs, annual/perennial; stinging hairs +/(0); leaves usu. only basally opposite; C quincuncial, (connate), not clawed; A (adnate to C), centripetal; stigma lobed; testa striate; n = (?19-)21.
1/15. S.W. North America. [Photo - Eucnide Flower © J. Reveal.]
[Schismocarpus [Loasoideae [Mentzelioideae + Gronovioideae]]: ?
2. Schismocarpus pachypus S. F. Blake
Shrublet, with xylopodium; leaves spiral; C not clawed; A = 2 X C, obdiplostemonous, filaments shorter than the anthers; nectary 0; G opposite C, semisuperior, stigma lobed; testa striate; n = ?
1/1. S. Mexico.
[Loasoideae [Mentzelioideae + Gronovioideae]]: (wood rayless); (inflorescence with con-/recaulescence [= metatopy]); G [3-5], when [3], odd member adaxial.
Age. The age of this node is estimated to be (86.4-)76.7-72.2(-62.1) Ma (Acuña Castillo et al. 2019).
3. Loasoideae Gilg
(Iridoids 0); petals cymbiform/boat-shaped, clawed; A centripetal and centrifugal, in 5 groups opposite and enclosed by C (2 x C), movement to the centre of the flower, antesepalous staminodes + [outer whorl 3-4, connate, as complex nectariferous scales, inner whorl 2-5, separate]; pollen ?not striate; capsule (spirally twisted), K and C shed separately; x = 6.
14/230. Mostly America, some Africa and the Marquesas Islands.
Age. Crown-group Loasoideae are estimated to be (61.9-)54.7, 52.1(-44.8) Ma (Acuña Castillo et al. 2019) or (63.0-)56.0, 53.1(-46.0) Ma (Acuã-Castillo et al. 2021).
3A. Klaprothieae Gilg
Shrubs (annual/perennial herbs), (deciduous); rootstock various; leaves opposite (spiral above), lamina venation ± palmate; flowers usu. 4-merous; C (smaller than K), often with 2 longitudinal lamellae; A movement autonomous; G semi-inferior; capsule (dehiscing longitudinally, valves opposite K), (indehiscent, K accrescent, seeds 1-2 - Kissenia); seeds usu. striate, at least equatorially, (papillose-reticulate); n = 12 (18).
3/6 or 4/9. South America, Africa, also the Arabian Peninsula (Kissenia, if included) and the Marquesas Islands (Plakothira). Photo: Flower.
Age. Klaprothieae (not including Kissenia) are estimated to be (36.8)29.6-27.8(-21.7) Ma (Acuña Castillo et al. 2019) or ca 28 Ma (Martín et al. 2022).
3B. Loaseae Reichenbach(Annual) herbs to shrubs, (deciduous); stinging hairs +, (uniseriate hairs - Nasa); leaves opposite (and spiral, esp. above), lamina ± palmate to pinnate, margin often deeply lobed; (inflorescences axillary); flowers usu. 5(-8)-merous; A movement thigmotactic (not); capsule (longitudinally septicidal/septifragal); testa (several cell layers across), (exotestal cells anticlinally elongated, walls fenestrate); n = 6-8, 12-15, 18...
9/221: Nasa (105), Caiophora (56), Loasa (36). Chile and Argentina to S. Mexico, Hispaniola. [Photo: Flower, Flower, Flower, Fruit].
Age. Crown-group Loaseae are around (56.5-)50.1-47.8(-41.3) Ma (Acuña Castillo et al. 2019).
[Mentzelioideae + Gronovioideae]: (hypanthium +); loss of C-A synorganisation.4. Mentzelioideae Fenzl - Mentzelia L.
Annual or perennial herbs to small trees, (deciduous); K and C quincuncial, shed as a unit, C not clawed; A centripetal, connate basally, (10), (staminodes +, petal-like/forked); nectary 0; stigma punctiform; n = 7, 9(-11).
1/80. The Americas, the Caribbean Islands and the Galapogas. Photo: Flower © S. Wolf.
5. Gronovioideae (Reichenbach) Link —— Synonymy: Cevalliaceae Grisebach, Gronoviaceae Endlicher
Scandent annuals, lianes, shrubs; (stinging hairs +); (inflorescence a raceme); (bracts recaulescent); C valvate, (connate), often smaller than K, with a single vascular trace, clawed or not; A 5, opposite K, (A 2, three staminodes - some Petalonyx), (connective long-clavate); (pollen tectum various); G [2-5], stigma ± lobed; ovule single, apparently apical, ?crassinucellate [Petalonyx, Gronovia], (funicular obturator +); fruit a cypsela, K accrescent, (deciduous, bract and bracteoles attached - Petalonyx); testa none; endosperm haustoria 0; n = 13, 23, 37.
4/9: Petalonyx (5). N.W. Peru to S.W. U.S.A., Hispaniola. Photo: Gronovia Flower.
Evolution: Divergence & Distribution. Schenk and Hufford (2008) suggest dates for some of the main clades in the family, and for more ages in Loasoideae, see Acuña Castillo et al. (2019).
Loasaceae as a whole may have originated in the southwest U.S.A-Mexican area, and crown-group Loasoideae are centred on the tropical Andes and the adjacent arid Pacific coast. Subsequent diversification of the Loasoideae seems to be associated with the orographic history of the Andes (Acuña Castillo et al. 2019 and references). Some species of all of the four clades that make up Nasa are to be found in the Amotape-Huancabamba zone (Acuña-Castillo et al. 2021); the crown-group age of this genus is around 30 Ma, but there is a phylogenetic fuse of ca 20 Ma. C. M. Martin et al. (2022) note a major disjuction (ca 1,500 km) within Xylopodia, and also a trans-Andean disjunction.
The distribution of the [Plakothira + Klaprothia + Kissenia] clade, Loasoideae-Klaprothieae (Hufford et al. 2005) is remarkable - the Marquesas, South America, N.E. and S.W. Africa and Arabia... (see also Acuña-Castillo et al. 2019).
Ecology & Physiology. For the morphology and mineral composition of the stinging hairs of Blumenbachia and other taxa, see Mustafa et al. (2017: Loasaceae hairs in general, 2018b; also Ensikat et al. 2017). Both the glochidiate/scabrid hairs (not fundamentally different) and the stinging hairs are unicellular. Mentzelia pumila, covered in glochidiate and spiky christmas tree-like hairs, traps a variety of insects, perhaps taking up nitrogen from them; the aphid, Macrosiphon mentzelieae, nevertheless seemed to be undeterred by these hairs, but was eaten by the coccinellid Hippodamia convergens that was, however, quite often (20%) also trapped (Eisner et al. 1998).
Pollination Biology. There are some very distinctive floral morphologies in the family and a variety of pollinators, although pollination by short-tongued bees may be the plesiomorphic condition for Loasoideae, at least (Henning et al. 2018). Weigend and Gottschling (2006) discuss pollination in Nasa; there are revolver flowers in this genus (see also Weigend et al. 2003 for nectaries, etc.). Pollination of some South American taxa by small mammals is likely, and in general nectar amount and sugar concentration increase with altitude (Ackermann & Weigend 2006). Henning et al. (2018) studied the remarkable thigmonastic pollination mechanisms found in many Loaseae, where stamen movement was triggered as the pollinator probed the nectar-containing staminodes (the nectar is secreted on the receptacle), the stimulus each time resulting in one or a few stamens moving to the centre of the flower. Mittelbach et al. (2019) suggest that in Nasa poissoniana at least the flower may respond to regularly-visiting (in terms of times separating visits) insects by allowing stamens to move to the centre of the flower independently of any stimulus in anticipation of the pollinator's visit. Petaloid staminodes in Mentzelia section Bartonia may improve bee visitation (but Apis mellifera the bee), yet they have been lost about as many times as they have been gained (ca 7 times: Botnaru & Schenk 2010).
Plant-Animal Interactions. Loasaceae from drier environments and in danger of being eaten by mammals tend to contain a diversity of iridoids; interestingly, genera like Nasa that can be defoliated by pyralid caterpillars have little in the way of iridoids, but Nasa in particular has a great diversity of leaf shapes (Weigend et al. 2000).
Genes & Genomes. Poston and Tompson (1977 and references) and Grau (1988) provide some chromosome numbers.
Chemistry, Morphology, etc.. There is variation in the composition of fatty acids in the seeds but the systematic significance of this is unclear. Of the taxa studied by Weigend et al. (2004b), Nasa (Loasoideae) was most distinct from this point of view; it is well embedded in the family.
Fernández Honaine et al. (2023) discuss cystoliths/silica-calcium-phoshorus deposition in this group.
For inflorescence morphology and concaulescence and recaulescence of bracts, etc., see Weigend (2004 and references). In some species of Petalonyx (Gronovioideae) there is postgenital fusion of the corolla, this forces the stamens outside the corolla whorl. For the complexities of androecial initiation, see Hufford (1990); antepetalous stamens arise from the flanks of primordia of antisepalous stamens. Hufford (2003) described staminode evolution in detail; see also Weigend et al. (2003) for the staminodes of Nasa, = nectar scales. The stigma is at least sometimes very long (Loasa triphylla - Hanf 1935). Although the ovule of Petalonyx is apparently apical, its "origin is distinctly lateral" (Hufford 1989b: p. 223), i.e., the placentation is basically parietal. Garcia (1962, 1963 and references) found both the chalazal and micropylar endosperm haustoria to be very variable, much branched or not - the chalazal haustorium was single- to many-celled, the micropylar endosperm haustorium coenocytic. The young endosperm of Blumenbachia had ca 8 cells in a linear series, the two micropylar cells being elongated and binucleate, the chalazal cell being uninucleate and with haustorial branches (Garcia 1963).
Additional information is taken from Moody and Hufford (2000a) and Weigend (2004: general), Thompson and Ernst (1967: Eucnide), Rodriguez et al. (1997, 2002) and Weigend et al. (2000), all iridoids, Carlquist (1984: wood anatomy), Leins and Winhard (1973), Brown and Kaul (1981), Weigend (1996) and Hufford (1988a, 1989a, 1990), all floral morphology/development, and Hufford (1988b) and Weigend et al. (2005), testa morphology.
Phylogeny. Strongly supported relationships suggested by Moody and Hufford (2000a), Moody et al. (2001), Hufford et al. (2003) and Hufford (2003) are Eucnide [Schismocarpus [Loasoideae [Mentzelioideae + Gronovioideae]]]. Within Loasoideae, the clade [Plakothira + Klaprothia + Kissenia] may be sister to the rest, but that relationship has little support (Hufford et al. 2005), or the clade may be part of a major polytomy (see also Weigend et al. 2004a). Henning et al. (2018) recovered a quite well supported Loaseae, a poorly supported Klaprothieae, in which Kissenia was sister to the well supported clade making up the remainder of the tribe; the position of Huidobria as sister to the rest of the subfamily had little support. Acuña Castillo et al. (2019) found that both Kissenia and Huidobria were at the very base of the clade that included Klaprothieae, this whole clade being sister to Loaseae, whose relationships were examined in detail; there was a polytomy here in Acuña Castillo et al. (2021), But Nasa was the centre of attention. Acuña et al. (2017) discussed relationships in southern Andean Loaseae.
Acuña Castillo et al. (2021: plastid genes) found four main clades in their well-samples analysis of Nasa; the old series did not hold up, while in a hierarchical cluster analysis members of three of these clades each largely corresponded to a single cluster, while the fourth was very heterogeneous.
Classification. The classification above clearly leaves a lot to be desired in terms of comprehensiveness. It is based on early molecular studies in which the whole family was sampled (see above), but it does not agree so well with morphological data (Weigend 2004). For some generic limits in Loaseae, see Acuña et al. (2017).
Previous Relationships. In older classifications Loasaceae were often grouped with other families that had parietal placentation (e.g. see Cronquist 1981).