EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing a vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
Evolution: Possible apomorphies for flowering plants are in bold. The actual level at which many characters, particularly the more cryptic ones, should be assigned is unclear. This is because some taxa basal to the [magnoliid + monocot + eudicot] group have been surprisingly little studied, there is considerable homoplasy as well as variation within and between families of the ANITA grade in particular for several of these characters, and also because details of relationships among gymnosperms will affect the level at which some of these characters are pegged. For example, if reticulate-perforate pollen is optimized to the next node on the tree (see Friis et al. 2009 for a discussion), it effectively makes the pollen morphology of the common ancestor of all angiosperms ambiguous... For other features such as a nucellus only one (Nymphaeales) to three cells thick above the embryo sac and a stylar canal lacking an epidermal layer, although plesiomorphous for basal grade angiosperms (Williams 2009), where on the tree a thicker nucellus and a stylar epidermal layer are acquired has not yet been indicated.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: (benzylisoquinoline alkaloids +); sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[MONOCOTS [CERATOPHYLLALES + EUDICOTS]] - if the clade exists: (veins in lamina often 7-17 mm/mm2 or more [mean for eudicots 8.0]); (stamens opposite [two whorls of] P); (pollen tube growth fast).
EUDICOTS / eudicotyledons, tricolpates - Back to Main Tree
(Myricetin +), asarone 0 [unknown in some groups, + in some asterids], ethereal oils usu. 0; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal [male meiotic cytokinesis unidirectional], wall with endexine; (megaspore mother cells several); G with complete postgenital fusion, stylulus/style solid [?here], short [≤2 x length of ovary]; seed coat?; palaeotetraploidy event; FLC gene lineage +.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. Note that the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Estimates of the age of the crown eudicot clade commonly range from 150-120 Ma, e.g. ca 146 Ma (Zeng et al. 2017, c.f. Foster & Ho 2017), (154-)145(-136) Ma (Foster et al. 2016a), (153-)147, 131(-125) Ma (Wikström et al. 2001, 2004), (167.3-)149(-128.4) Ma (Tank & Olmstead 2017: note topology), ca 135 Ma (Tank et al. 2015: Table S1), 122-120 Ma (Anderson et al. 2005), (123.9-)122.2(-116.8) Ma in Schwery et al. (2014), ca 125 Ma (Magallón & Castillo 2009), (145-)130, 129(-117) Ma (or thereabouts: Bell et al. 2010), (127-)126(-123) Ma (N. Zhang et al. 2012; see also Xue et al. 2012), and 125-119.5 Ma (Morris et al. 2018), and for similar estimates see P.-L. Liu et al. (2020). Soltis et al. (2008: several estimates) suggest an age for crown eudicots of 152-110 Ma, however, the age in Z. Wu et al. (2014) is around 197 Ma while it was only 128-124 Ma in Foster and Ho (2017); a rather unlikely age of (100.4-)96.3(-93) Ma is suggested by Iles et al. (2014). A variety of ages is given by Christin et al. (2014a), but topology? See also Guindon (2018). H.-T. Li et al. (2019) estimate an age of (161-)132(-125) Ma, Strijk et al. (2019) an age of (136.2-)130.2(-126.6) Ma, P.-L. Liu et al. (2020) an age of (140-)136(-129) Ma, X. Guo et al. (2021) an age of ca 138.9 Ma, Y. Yang et al. (2020: Suppl. Fig 22) an age of around 126.3 Ma and L. Yang et al. (2020) an age of 131.1-127.8 Ma. (Ages in Bell et al. 2005; Moore et al. 2007; S. A. Smith et al. 2010; Clarke et al. 2011; Magallón et al. 2013, 2015; Zanne et al. 2014; Zeng et al. 2014; Beaulieu et al. 2015; Murat et al. 2017; Foster et al. 2017, and Barba-Montoya et al. 2018 - see H.-T. Li et al. 2019: table 2 - are 188-187 Ma.) The age in Y. Li et al. (2020) is ca 122.1 Ma, in Jiao and Wang (2022) 138-122 Ma and in Peng et al. (2023a) (145.0-)142.7(-140.9) Ma.
Tricolpate pollen has been found in the Late Barremian-Early Aptian of the Cretaceous some 127-120 Ma, and so a minimum age of some 125 Ma for the eudicots is reasonable (e.g. Doyle & Hickey 1976; Magallón et al. 1999; Sanderson & Doyle 2001; Friis et al. 2011; Herendeen et al. 2017), about the age of the oldest monocot fossils. However, S. A. Smith et al. (2010) note that when tricolpate pollen first appears in the fossil record it is both widely dispersed geographically and quite heterogeneous (see also Friis et al. 2006b), and this might imply an earlier origin of the clade, the fossils then being more marks of its "rise to dominance" than of its origin (Beaulieu et al. 2013: p. 4).
The recent discovery of Leefructus, dated to at least 122.6 Ma old and assigned to stem group Ranunculaceae (G. Sun et al. 2011; c.f. Crepet et al. 2004 for an earlier mesofossil estimate), could also imply a substantially greater age for Ranunculales - and hence the whole eudicot clade - of ca 152-140 Ma (simple extrapolation from the ages of various clades in Ranunculales given by Anderson et al. 2005; c.f. W. Wang et al. 2014a, 2016b below). Although Leefructus seems quite well preserved, it is not associated with pollen (Sun et al. 2011). Friis et al. (2011, see also 2017a) discuss a variety of other early fossils that are, or from general morphology might be, associated with plants with tricolpate grains.
Evolution: Divergence & Distribution. There is a substantial period of ca 34 Ma between the [[Ceratophyllales + Chloranthales] eudicots] node and the beginning of eudicot diversification (Zeng et al. 2014). Subsequent divergence of eudicot clades like Proteales, Buxales, etc., may have been rapid, occurring 120-116 Ma (Anderson et al. 2005), while Wikström et al. (2001) thought that the clades immediately below core eudicots had diverged by (140-)135, 123(-118) Ma.
Doyle (2007) scored chloranthoid teeth as plesiomorphous for eudicots; given current ideas of phylogeny, they may be an apomorphy here. He also considered palmate-crowded veins to be an apomorphy for all eudicots, but Sabiaceae were not mentioned, and the interpretation of the venation of Euptelea is debatable, as he noted (Doyle 2007). The palmate venation in aquatics like Nelumbo may further confuse the situation; palmate venation is common in aquatics with their broad peltate or cordate-based leaf blades and so is associated with the aquatic life style.
Sauquet et al. (2017: see Supplementary Fig. 4) note how difficult it is to reconstruct the morphology of the flower at this node. There is a valuable survey of floral morphology of the whole eudicot clade in Endress (2010c: p. 540), representing "a first [sic] attempt to characterize the major subclades of eudicots". Characterizations there are a mixture or apomorphies and plesiomorphies, with an emphasis on "tendencies". J. Ma et al. (2021) noted that the FLOWERING LOCUS C (FLC) gene, a transcriptional regulator that i.a. represses the transition to flowering, was to be found only in eudicots, but not in Ceratophyllaceae, etc..
Dimerous flowers are to be found in the basal eudicot grade, but are uncommon in taxa at the nodes above core eudicots and in monocots (Drinnan et al. 1994; Soltis et al. 2003; Wanntorp & Soltis et al. 2005; Ronse De Craene 2005; Doust & Stevens 2005; Kramer & Zimmer 2006; Moody & Les 2007; Doyle 2013), and such flowers are also found in the core eudicot Haloragaceae); Endress (2010c) also emphasized that the flowers may be trimerous. Indeed, it has been suggested that the pentamerous flowers of Sabiaceae (Proteales) are derived from trimerous ranunculalean flowers, there being some kind of relationship between the two groups (e.g. Endress 2010c; Ronse De Craene et al. 2015a; c.f. Züñiga 2015) which perhaps have apomorphic tendencies in common (Ronse De Craene et al. 2015b). Stamens are also quite often inserted opposite the tepals in the basal eudicot grade, even if there is more than a single whorl of tepals (e.g. see Endress 1995a for illustrations of these in Ranunculales; Doust & Stevens 2005). This feature is placed at the [monocots [Ceratophyllales + eudicots]] node here, but the flowers of Lauraceae (magnoliids) are similar in this respect.
Taxa with androecia that are initiated as antesepalous triplets are scattered throughout the group (Hufford 2001a), if rather uncommon. Although stamen number may be high, development is rarely simply centripetal, as in Magnoliales (e.g. Corner 1946b), and carpel and perianth/petal number do not often increase in parallel, unlike in the euasterids. The basic pollen type for eudicots seems to be tectate/semitectate-reticulate, the latter grains being found in e.g. Platanacaeae, Menispermacaeae, Hamamelidaceae, Gunneraceae (Denk & Tekleva 2006) and Nelumbonaceae; see M.-Y. Zhang et al. (2017) for pollen evolution in the basal eudicots. Matomoro et al. (2015) discuss the evolution of pollen morphology/morphogenesis in terms od selection pressures and developmental constraints; they note that when apertures are not equatorial, pollen morphology and development in particular vary considerably. For an optimisation of syncarpy in this part of the tree, see Sokoloff et al. (2013d). Taxa with multicellular archesporia (= "megaspore mother cells several") are scattered throughout this clade, but nowhere else in angiosperms apart from Laurales (Vinogradova 2022: esp. Fig. 2).
The evolution of giberellin receptors is of considerable interest in this part of the tree, the receptor(s) being different from those in other land plants (Yoshida et al. 2018), although sampling needs to be improved to work out what is going on. Thinking about cellulose synthesis, xylans are more common than glucomannans, as in other angiosperms. There are glucoronosyl units (α1,2-MeGlcA) every 6 or 8 or so xylosyl residues, and every other xylosyl residue is acetylated, as are the glucoronosyl units themselves, and there are no α-arabinosyl units (Busse-Wicher et al. 2016). This is not that different from what is going on in magnoliids, and exactly where any changes in cell wall synthesis should be placed on the tree is unclear. Triterpenoids produced by a variety of CYP716 enzymes throughout this clade (Miettinen et al. 2017). Cuticle waxes as clustered tubules, nonacosan-10-ol the main wax, could perhaps be optimised to this position, later being lost in Sabiaceae, Platanaceae, Buxales (but in Buxus), and perhaps also in core eudicots (such waxes are present in a few Santalales, also in woody Saxifragales: see Barthlott et al. 2003).
Ecology & Physiology. Liu et al. (2014) suggest that it is only with eudicots that we see generally faster litter decomposition, with all the implications this has for nutrient cycling (see below. Another set of important changes that can perhaps be placed around here has to do with an interrelated set of features like photosynthesis, venation density, and stomatal and genome sizes, and this is also discussed elsewhere.
Pollination Biology. Diversification of eudicots is roughly contemporaneous with that of bees; the latter is estimated to have begun (132-)123(-113) Ma (Cardinal & Danforth 2013). For fossils of flowers perhaps pollinated by bees, see above.
Protandry is common in eudicots, although aquatic taxa tend to be protogynous, and protogyny - rather, interfloral protogyny - is also common in mono- and dioecious taxa (see Bertin & Newman 1993).
Dajoz et al. (1991), Halbritter and Hesse (2004), Furness and Rudall (2004) and Matomoro-Vidal et al. (2015) discuss the possible functional significance of the evolution of triaperturate pollen and of pollen apertures in general; the occurrence of several apertures on one grain may increase the speed of germination of the pollen, but at the same time decrease its viability and affect its harmomegathic movements. There appears to be a fixed developmental sequence for the pollen of core eudicots that allows the development of particular pollen "types" (Ressayre et al. 2002).
The Eudicot Evolutionary Research website should also be consulted.
Genes & Genomes. Murat et al. (2017) suggested that the ancestral eukaryote karyotype had seven protochromosomes. Recent work has attempted to clarify earlier suggestions that the γ whole nuclear genome duplication, a palaeohexaploid event (also known as the gamma triplication) might be made up of two events, a tetraploidy event to be placed here and an allopolyploidy event at the Gunnerales node, i.e. 4x x 2x (Aköz & Nordborg 2019). However, P.-L. Liu et al. (2020) thought that a whole genome duplication in Aquilegia coerulea had nothing to do with other duplications in this area, as did Shi and Chen (2020), the latter suggesting that that duplication was within Ranunculales, and closer than Papaver - but see reply by Aköz and Nordborg (2020). For more discussion of these events, see the core eudicot node.
Salse et al. (2009) suggested that the common ancestor of the eudicots had seven chromosomes, but this estimate needs to be revisited - 14, or something else?
Around five times as many gene families expanded at this node compared to the core eudicot node (551 versus 110), while only half the number contracted (P.-L. Liu et al. 2020).
There is a ycf68 pseudogene practically through the eudicot clade, with the exception of Citrus aurantiifolia (H.-J. Su et al. 2014). For the gene content of the inverted repeat in the immediate ancestor of the eudicots, see Y. Sun et al. (2015).
Taxa in which GLO-like proteins cannot form heterodimers predominate in this clade (Melzer et al. 2014); DEF-like proteins also cannot do this (see also above). Melzer et al. (2014) suggest that this may contribute to the increasing canalization of floral development.
Chemistry, Morphology, etc.. See Hegnauer (1990) for a discussion of the chemistry of the old Polycarpicae, which has turned out to be a grade group including many Ranunculales, the magnoliids and Austrobaileyales. The Cellulose Synthase gene superfamily is involved in synthesising components of the cell walls, and of these, CslB (sister to the monocot CslH) and CslM (sister to CslJ) can be placed at this node (Little et al. 2018).
Phylogeny. Ranunculales are usually sister to all other eudicots, and in some trees Ceratophyllaceae are sister to eudicots (e.g. Moore et al. 2007); see also the discussion at the Mesangiospermae node. The relationships between Chloranthales, magnoliids, Ceratophyllales and monocots, which will all be immediately basal/sister to the eudicots, have been unclear for some time.
Eudicot relationships somewhat different from those in APG IV are suggested by Zuntini et al. (2024). In APG IV (2016) they are [Trochodendrales1 [Buxales2 [Gunnerales3 [[Saxifragales4 [Vitales5 [[Zygophyllales6 [Celastrales7 [Oxalidales8 + Malpighiales9]]] [[Fabales10 [Rosales11 [Fagales12 + Cucurbitales13]]]] [[Geraniales14 + Myrtales15] [Crossosomatales16 [Picramniales17 [Sapindales18 [Huerteales19 [Brassicales20 + Malvales21]]]]]], Dilleniales22, [Berberidopsidales23 [Santalales24 [Caryophyllales25 [[Cornales26 [Ericales27 [[Icacinales28 [Metteniusiales29 [Garryales30...]]] [Aquifoliales31 [Asterales32, Escalloniales33 [Bruniales34 [Apiales35 [Dipsacales36 + Paracryphiales37]]]]]]]]]]]]]]]]]]] but ...[[1 + 2] *[*[3 + 22] [25 *[24 [5 [4 [[14 + 16] *[*[6 + 15]] [[[10 + 12] [11 + 13]] [17 [[7 [Apodanthales (ex 13) *[9 + Huales (ex 8)]]] [19 [8 [18 [20 + 21]]]]] [23 [26 + ×27] [Cardiopteridales (ex 28) *[28 *[31 + 30]]]] [[Oncothecales (ex 28) + 29] [34 *[32 [35 *[Columelliales (ex 34) *[36 [33 + 37]]]]]]]]]]]]]]]], "*" = nodes with posterior probablities (much) less than 0.9.
There is some uncertainty about basal eudicot relationships. A [Buxales + Trochodendrales] clade was recovered by Zuntini et al. (2024). An unresolved Proteales and Sabiaceae are often sister to eudicots minus Ranunculales (e.g. S. Kim et al. 2004b). A position [Ranunculaceae [Sabiaceae [all other eudicots]]] had only 83% support, of which most came from the matK gene (the other genes examined were petD and trnL-F) in analyses by Worberg et al. (2006, 2007); for this topology, see also Qiu et al. (2010: support weak). A three-gene analysis by Soltis et al. (2003) also found that that Sabiaceae were near Proteales, Buxales, etc., while Morton (2011: nuclear Xdh gene) found some support for a [Platanaceae + Ranunculales] clade and a four gene analysis (Kim et al. 2004a) had a weakly supported [Trochodendrales [Sabiaceae + Buxales]] clade. Moore et al. (2008) did not find strongly-supported relationships in this part of the tree, and various permutations of relationships of the groups being discussed, none strongly supported, were found by Zhu et al. (2007). Soltis et al. (2008) used the topology [Proteaceae [Sabiaceae [Buxaceae [Trochodendraceae + core eudicots]]]] in their book, a topology also recovered by Z.-D. Chen et al. (2016), while [Proteaceae [Sabiaceae ....]] relationships were found by Gitzendanner et al. (2018a) - see also Goloboff et al. (2009) and Fiz-Palacios et al. (2011) for other relationships.
Chase et al. (1993) and Drinnan et al. (1995) had found Platanaceae and Nelumbonaceae to be sister taxa; a close relationship was confirmed in the chloroplast genome analysis of Xue et al. (2012). A Proteales s. str. (Nelumbonaceae, Platanaceae and Proteaceae) and Sabiaceae are sister taxa in an analysis of all 79 protein-coding plastid genes and four mitochondrial genes (Moore et al. 2008: support only moderate; see also Soltis et al. 2011 and Moore et al. 2011: support weak in both cases). The two were also sister in the major analyses of chloroplast and nuclear data in M. Sun et al. (2014), but not in the mitochondrial study and in many of the supplementary trees; support was strong in the analysis of Y. Sun et al. (2015). Savolainen et al. (2000a), Qiu et al. (2006b, c.f. 2010), Burleigh et al. (2009), N. Zhang et al. (2012), Y.-x. Sun et al. (2013), Ruhfel et al. (2014: not all analyses), Z. Wu et al. (2014), Magallón et al. (2015), Barba-Montoya et al. (2018) and T. Yang et al. (2018: chloroplast data, other hypotheses of relationships rejected) have also found evidence for an association of Sabiaceae with Proteales, although support qas sometimes weak. This grouping was also recovered, and with quite strong support, in the i.2022 version of Seed Plant Tree and so an expanded Proteales is recognised here (see also A.P.G. IV 2016), but c.f. H.-T. Li et al. (2021) who inclined towards the recognition of a monofamilial Sabiales. Morphology is consistent with the inclusion of Sabiaceae in Proteales.
The relationships of Buxales and Trochodendrales are also unclear, hence the tritomy in the main tree. Qiu et al. (2006b) found uncertain relationships in a three-gene analysis of mitochondrial data, but with eight genes a topology [Buxales + core eudicots] was recovered (see also Qiu et al. 2010; Hilu et al. 2003; Wikström et al. 2003). Soltis et al. (2011) in their seventeen-gene analysis also found strong support for the [Buxales + core eudicots] clade (see also Moore et al. 2010; Xue et al. 2012; Vekemans et al. 2012; Y.-x. Sun et al. 2013; Ruhfel et al. 2014; Z. Wu et al. 2014; Magallón et al. 2015; Foster et al. 2016a; Barba-Montoya et al. 2018; T. Yang et al. 2018: chloroplast data; H.-T. Li et al. 2019, 2021, both plastomes, support weak; Gitzendanner et al. 2018b; Peng et al. 2023a). In a 3-gene (chloroplast) phylogenetic analysis focussing on the eudicots with a similar combined-data topology (Worberg et al. 2006, esp. 2007a), individual trees showed a variety of relationships. Most of the relationships they found along the eudicot spine were strongly (>90% jacknife) supported. However, Y. Sun et al. (2015) obtained the relationships [Trochodendrales + core eudicots] in their plastome study, but with around only 55% ML bootstrap support. Such relationships had sometimes been obtained before, for example, with strong support by Moore et al. (2011), and also in a few early studies (see Y. Sun et al. 2015 for references). Finally, Gitzendanner et al. (2018a) obtained a weakly-supported [Buxales + Trochodendrales] clade sister to the eudicots (and see also J. F. Walker et al. 2019: relationships depend on genes analyzed; W. J. Baker et al. 2021: see Seed Plant Tree, Angiosperms353 nuclear genes).
Classification. The names "eudicots" and "eudicotyledons" were apparently first used by Doyle and Hotton (1991), and they seem to be informal - but they are very useful. Wikipedia mentions a "Eudicotidae" - perhaps "eudicot' with a subclass termination, but I had not come across this before viii.2024...
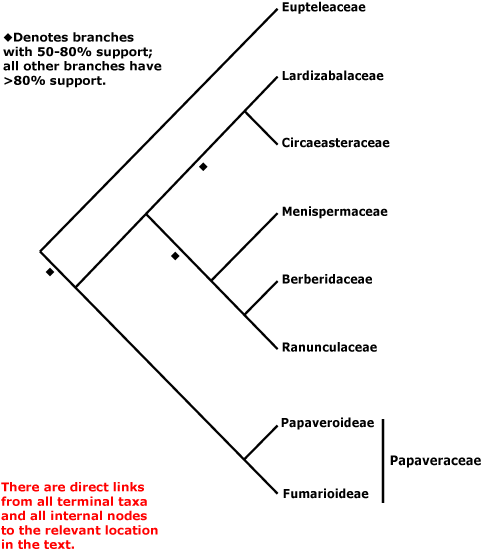
RANUNCULALES Berchtold & J. Presl - Main Tree.
(O-methyl)flavonols, flavonols, isoquinoline alkaloids [?here] +; vessel elements?; young stem with separate bundles, vessels only in central part of bundles, true tracheids +; rays exclusively wide multiseriate [and in wood, where present, composed mostly of procumbent cells]; (wood fluorescence +); cambium storied; sieve tube plastids large S-type, dispersive P-protein +; petiole bundles annular; leaf cuticle waxes as clustered tubules with nonacosan-10-ol the main wax [?Euptelea]; leaves spiral, lamina serrate, ?tooth morphology; G opposite P, style 0; ovules 1-2/carpel, bistomal; P deciduous in fruit; seed exotestal; endosperm development?, embryo size?; whole genome duplication. - 7 families, 199 genera, 4510 species.
Includes Berberidaceae, Eupteleaceae, Circaeasteraceae, Lardizabalaceae, Menispermaceae, Papaveraceae (inc. Fumarioideae, Papaveroideae), Ranunculaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. Note that the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown-group Ranunculales may be (146-)140, 126(-120) Ma (Wikström et al. 2001: c.f. topology); Anderson et al. (2005) dated them to 121-114 Ma, Magallón and Castillo (2009) to ca 113.2 Ma, while about 114.8 Ma is the estimate in Magallón et al. (2015), about 132.5 Ma that in Tank et al. (2015: Table S1, S2, relationships at base unclear), (123-)116.0(-111.8) Ma by Y. Sun et al. (2020: note relationships) and (138.3-)136.3(-134.5) Ma (Peng et al. 2023a).
Magallón et al. (1999) suggested a fossil-based age of ca 70 Ma for the whole clade, but the fossil was a member of the decidedly non-basal Menispermaceae. Teixeiraea is a name given to multistaminate staminate flowers from the Early Cretaceous of Portugal - the stamens are of two sizes and have tricolpate pollen - that can perhaps be assigned to crown-group Ranunculales, but these fossils might rather belong to Berberidopsidales or Saxifragales (von Balthazar et al. 2005). Paisia, a late Aptian/early Albian fossil from Portugal ca 113 Ma has 5-merous flowers with perianth, stamens and conduplicate carpels all opposite each other and pantoporate pollen, and it, too, may belong around here (Friis et al. 2018). If the identity of Leefructus, a fossil assigned to stem group Ranunculaceae that is at least 122.6 Ma old (G. Sun et al. 2011), is confirmed, Ranunculales may be 152-140 Ma or so (extrapolating from the dating suggestions of Anderson et al. 2005, but see below).
Krassilov and Volynets (2008) discuss a number of fossils from the Early to Middle Albian (ca 105 Ma) of Primorye that they associated with Ranunculidae sensu Takhtajan, comparing some specifically with Ranunculaceae. The morphology of these fossils is odd, some appearing to have abaxially dehiscent follicles (c.f. Cercidiphyllaceae) and others have axillary fruits at nodes from which branches also arise. The plants are very small, and were described as being weedy (Krassilov & Volynets 2008). The Early Cretaceous Archaefructus has also been compared with Delphinium (Becerra et al. 2012), while Klitzschophyllities, around 110 Ma, probably was an aquatic; it is known from Brazil, Portugal, and North Africa and may be stem Ranunculales (Gomez et al. 2009).
Evolution: Divergence & Distribution. Anderson et al. (2005) noted that all ranunculalean families had diverged before 105 Ma except Ranunculaceae/Berberidaceae, where divergence occurred 104-90 Ma (see also Wikström et al. 2001). However, the recent discoveries of Potomacapnos, ca 120 Ma from the eastern U.S.A., Papaveraceae-Fumarioideae (Jud & Hickey 2013) and Leefructus, 125.8-123 Ma from China, stem Ranunculaceae (G. Sun et al. 2011; W. Wang et al. 2014a, b) may suggest a different scenario. They suggest a very rapid diversification within Ranunculales; tricolpate pollen is first known from 127-125 Ma, or slightly earlier, and the thought is that all families in the order must have diverged within 5 Ma and so had appeared by ca 123 Mya - the "accelerated angiosperm evolution" of Wang et al. (2016b: p. 338).
Flowers in Ranunculales show considerable variability, and Damerval and Becker (2017) summarized what was known about floral development. Carrive et al. (2020) discuss i.a. the evolution of the perianth, suggesting that the ancestral condition was to have three trimerous petal-like whorls, although the outer whorl was not petal-like in some reconstructions. Subsequently there were both increases and decreases in the number of the whorls, and also sometimes assumption of a nectariferous fuction - this last especially in core Ranunculaceae. Carrive et al. (2020: Figs 3, 4, see also Table 1) also optimized several floral characters on the tree. Indeed, there has been considerable discussion over the identity of the different petal/tepal/sepal/stamen parts of the flower in Ranunculales (Carrive et al. 2020 for a summary). Almost all Ranunculales, perhaps minus Euptelea, have petals, that is, more or less expanded and attractive parts of the flower (Rasmussen et al. 2009). An inner, more or less petal-like, nectar-secreting whorl is especially obvious in many Berberidaceae and Ranunculaceae (but whether or not it is an apomorphy for the pair is unclear - Carrive et al. 2020), and is often interpreted as being derived from stamens. Drinnan et al. (1994) suggested that petals had been derived from stamens several times, and Glover et al. (2015) thought that they may also be lost. However, Sharma et al. (2014 and references) found no developmental evidence for a connection between more or less petal-like nectaries and stamens in Ranunculaceae, at least, and Carrive et al. (2020) also saw no immediate connection between the two.
Gene expression patterns in the inner perianth whorl of Ranunculaceae and Berberidaceae are unique, and intermediates can be explained by the fading boundaries model of development (ref.). Chanderbali et al. (2010) found that expression of genes active in each floral whorl in flowers of the one member of Ranunculales they examined (Escholtzia) were restricted to that whorl, as in other eudicots; within Ranunculales, Papaveraceae-Papaveroideae, to which Escholtzia belongs, have a perianth that is apparently made up of a rather conventional calyx and corolla. On the other hand, in Delphinium (Ranunculaceae) expression patterns of genes active in the two outer floral whorls were not sharply differentiated (Voelckel et al. 2011). I may call the outer whorl, "calyx", and the inner whorl, "corolla", but this is simply for descriptive purposes.
Monosymmetry has evolved at least twice in Ranunculales, and Cycloidea genes are involved. However, they are variously expressed in the flower, ad- or abaxially or laterally, and may also be expressed in the outer perianth whorl (Jabbour et al. 2014 and references: see Papaveraceae-Fumarioideae and Ranunculaceae below). This is unlike the consistent adaxial expression in Pentapetalae studied (Hileman 2014 and references). For floral development, see also Becker (2016).
See W. Wang et al. (2009: extensive morphological data matrix) for the evolution of characters optimised on to a tree with the same topology as that used here, although it is difficult to work out where a character such as 1-2 ovules/carpel should be placed - however, low ovule numbers are probably plesiomorphic in the order. Dulin and Kirchoff (2010) discuss wood, woodiness and their evolution in Ranunculales, M.-Y. Zhang et al. (2017) pollen evolution, and Armbruster et al. (2002) and X.-F. Wang et al. (2011) compitum development. There is clearly extensive variation in floral phyllotaxis in the order (Y. Zhang et al. 2023). Variation and evolution in seed shape within the order was the subject of a study by Martín-Gómez et al. (2019).
Ecology & Physiology. Liu et al. (2014) suggest that it is only somewhere around this node that the origin of angiosperm leaves that decompose rather fast can be pegged.
Pollination Biology. Endress (2010c) emphasized the several independant origins of wind and especially fly pollination in the clade. There are a number of reports of delayed fertilization (up to some two months or more after pollination) in members of Ranunculales, including Eupteleaceae, Circaeasteraceae, Lardizabalaceae and Ranunculaceae (Sogo & Tobe 2006d for references).
Floral nectar spurs have evolved four to six times in Ranunculales; they may be on members of the outer (Myosurus, Delphinium) or inner (Aquilegia ) perianth whorls, and be five (Aquilegia ), two (Dicentra) or one (Delphinium) per flower (Damerval & Nadot 2007). They may secrete nectar (Aquilegia ), contain one or two nectaries/"petals" (Delphinieae), or collect nectar secreted by nectaries on adjacent stamen bases (Corydalis), etc..
Plant-Animal Interactions. Ranunculales - perhaps especially Menispermaceae and Ranunculaceae - are little used as food plants of butterfly caterpillars (Ehrlich & Raven 1964), probably because alkaloids and other noxious compounds are common (but see Papaveraceae).
Vegetative Variation. Gleissberg and Kadereit (1999) discussed the evolution of leaf form in the order, with polyternate/acropetal/basipetal-pedate leaves perhaps being plesiomorphic. The glandular leaf teeth have a clear, persistent, swollen cap into which higher order lateral veins also run. What is the distribution of colleters?
Genes & Genomes. For the complex pattern of duplication of APETALA3 and FUL-like genes and their expression in Ranunculales, and of the MADS-box genes as a whole, see Sharma et al. (2011), Pabón-Mora et al. (2013) and Soza et al. (2016) and references. Where to put these duplications on the tree is unclear, but perhaps at the [Ranunculaceae + The Rest] node (see also Zahn et al. 2005b; Cui et al. 2006; Tank et al. 2015). Indeed, a genome duplication (the ARTHγ event) ca 136.9 Ma or ca 110 Ma has been associated with the order as a whole (Landis et al. 2018; J. He et al. 2022).
Chemistry, Morphology, etc.. See Hegnauer (1990) for a discussion of the chemistry of the Polycarpicae, which also includes the magnoliids and Austrobaileyales. The benzylisoquioline alkaloid berberin, common in Ranunculales (check Eupteleaceae), is synthesised via the tyrosine pathway; for isoquinoline synthesis, including that of opium, see Y. Li et al. (2020). Leaf cuticle waxes as tubules, nonacosan-10-ol being an important component, are widespread in the order; I do not know if they are to be found in Euptelea.
Antipodal cells are commonly other than simply persistent; data are summarized in Williams and Friedman (2004).
For additional information, see Ernst (1964: general), Fay and Christenhuz (2012: illustrated summary), Hao et al (2018: chemistry and medecine), Hennig et al. (1994) and Barthlott and Theisen (1995: both cuticle waxes), Behnke (1995b: sieve tube plastids and phloem proteins), Carlquist and Zona (1988) and Carlquist (1995b), wood anatomy, Endress (1995a: floral morphology), Ronse Decraene and Smets (1995b: androecial variation), Blackmore et al. (1995: pollen, very variable), Brückner (1995: summary of seed anatomy), Floyd et al. (1999: embryology), and Floyd and Friedman (2000: endosperm).
Phylogeny. Relationships within Ranunculales are only moderately well understood - see Hoot and Crane (1995), Kadereit et al. (1995), Oxelman and Lidén (1995), Hoot et al. (1999: three genes), and Soltis et al. (2011). Soltis et al. (2003a: four-genes), Kim et al. (2004a), Worberg et al. (2006, 2007: non-coding chloroplast DNA), Y. Sun et al. (2018: plastome phylogenomics), Lane et al. (2018: phylogenomic analysis) and Sun et al. (2020: 264 gene analysis) suggest that Eupteleaceae may be sister to the whole of the rest of the order, although support for this position has sometimes been only moderate (e.g. Hoot et al. 2015; W. Wang et al. 2009: four genes, see also 2016b; support strengthened when morphological data were added; Sun et al. 2017). Some earlier studies have suggested other topologies, such as Ranunculaceae (Soltis et al. 2000; Hilu et al. 2008 - but no strong support for any position of Eupteleaceae) or Papaveraceae (Soltis et al. 2007a; Anderson et al. 2005; Bell et al. 2010; Z.-D. Chen et al. 2016: strong support) as sister to all other members of the order. There was very weak support for a [Eupteleaceae + Papaveraceae] clade in H.-T. Li et al. (2019) and this clade was also recovered in the genome analysis of Sun et al. (2020: 497 gene analysis, divergence (116.4-)105.9(-89.8) Ma, Menispermaceae not included), but in Li et al. (2021) there was some support for the relationships [Eupteleaceae [Papaveraceae ....]]. The clade [Circaeasteraceae + Lardizabalaceae] was strongly supported in the plastome analysis of Y. Sun et al. (2017), Li et al. (2021), etc.; see also Hoot et al. (2015: support variable). Furthermore, although a [Circaeasteraceae [Lardizabalaceae [Berberidaceae + Ranunculaceae]]] clade is sometimes recovered, in the analysis of Sun et al. (2020) the position of the first two is reversed; the [Berberidaceae + Ranunculaceae] clade is, however, generally recovered. Analysis of mitochondrial genes suggested a rather different basal set of relationships - [Menispermaceae [Papaveraceae [Eupteleaceae ...]]] (Qiu et al. 2010: Circaeasteraceae not included), although support was mostly (very) weak, that of the [Berberidaceae + Ranunculaceae] clade alone being strong. In an analysis of protein-coding chloroplast genes F. Wen et al. (2021) found that Circaeasteraceae were deeply embedded in Ranunculaceae, while at the other extreme are the basal relationships recovered by W. J. Baker et al. (2021: see Seed Plant Tree, Angiosperms353 nuclear data) — [Circaeasteraceae [Eupteleaceae ...]]. Indeed, Circaeasteraceae were found to be sister to all other Ranunculales by M. Guo et al. (2021: plastome analyses, Berberidaceae the focus), Berberidaceae being sister to the remaining taxa studied; this seems not to be a rooting problem. However, Peng et al. (2023a: plastid data) recovered the relationships [Eupteleaceae [Papaveraceae [[Circaeasteraceae + Lardizabalaceae] ...]]] and in the i.2023 version of the Seed Plant Tree relationships are [Eupteleaceae [Papaveraceae [Circaeasteraceae [Lardizabalaceae ...]]]].
Euptelea was placed well outside Ranunculales in purely morphological analyses and formed a clade with Nelumbo, Illicium, Paeonia, etc. - but mercifully without any bootstrap support (W. Wang et al. 2009); the topology was hightly pectinate, and very few branches had even poor bootstrap support, posterior probabilities being still worse. Loconte et al. (1995) had found Ranunculales to be paraphyletic in a morphological phylogeny.
Classification. For a classification of the order, largely followed here, see W. Wang et al. (2009).
Previous Relationships. A Papaverales, containing three families (= Papaveraceae below), were commonly recognised as a separate order next to Ranunculales (Cronquist 1981; Dahlgren 1989), but there is no point in recognising them, especially if Eupteleaceae are sister to all other Ranunculales.
Synonymy: Papaverineae Thorne & Reveal, Ranunculinae Bessey - Berberidales Berchtold & J. Presl, Circaeasterales Takhtajan, Eupteleales Reveal, Fumariales Link, Glaucidiales Reveal, Helleborales Nakai, Hydrastidales Takhtajan, Lardizabalales Loconte, Menispermales Berchtold & J. Presl, Nandinales Doweld, Papaverales Berchtold & J. Presl, Podophyllales Dumortier - Eupteleineae Shipunov, Lardizabalineae Shipunov - Berberidanae Doweld, Eupteleanae Doweld, Papaveranae Doweld, Ranunculanae Reveal - Ranunculidae Reveal - Berberidopsida Brogniart, Papaveropsida Brongniart, Ranunculopsida Brongniart
EUPTELEACEAE K. Wilhelm - Euptelea Siebold & Zuccarini - Back to Ranunculales
Deciduous trees; (dihydro)chalcones +; cork cambium deep in cortex; vessel elements with scalariform-reticulate perforations; rays to 10-seriate; nodes 1:5(-9); cuticle wax crystalloids 0; buds perulate; lamina vernation subplicate-conduplicate, margins gland-toothed, secondary veins pinnate; inflorescence axillary, fasciculate or umbellate; P 0; A 6-20, filaments short [much shorter than the anthers], anthers inconspicuously valvate, latrorse, connective prolonged; pollen (3-) 6-colpate, lamellate exinous oncus orbicules +; G 6-31, stipitate, "intermediate ascidiate", stigma brush-like, at most weakly secretory; ovules (-4/carpel), epitropous, outer integument 2-5 cells across, inner integument ca 2 cells across; antipodal cells do not persist; fruit a samara; exotestal cells ± enlarged, (mesotesta sclerotic), endotesta lignified, subpalisade; endosperm cellular; n = x = 14, nuclear genome [1 C] (0.068-)1.388(-28.205) pg; germination epigeal.
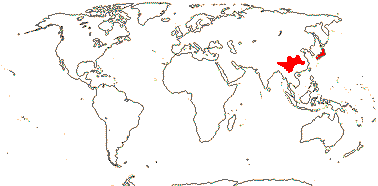
1 [list]/2. Temperate South East Asia (map: from Fu & Hong 2000). [Photo - Collection]
Age. Anderson et al. (2005) suggested an age of ca 120-111 Ma for stem-group Eupteleaceae, and Wikström et al. (2001) an age of (141-)135, 122(-116) Ma, but note topologies. Euptelea represents a very old and species-poor clade.
Evolution: Divergence & Distribution. Whatever its relationships, Euptelea is a very old and species-poor clade. Depending on the position of Eupteleaceae and the immediate relatives of the eudicots, stamen number here could represent an increase.
Chemistry, Morphology, etc.. Lateral veins only approach the glandular teeth; the gland itself has an apical cavity. Is the wood storied, what about fluorescence, separate bundles?
See Endress (1970a, 1993) for some general information, Hegnauer (1973, 1989, 1990) for chemistry, H.-F. Li and Ren (2005) for wood anatomy, Ren et al. (2007b) for floral development and Pérez-Gutiérrez et al. (2016) for pollen.
Previous Relationships. Eupteleaceae were placed next to Cercidiphyllaceae in Hamamelidales by Cronquist (1981) or Hamamelididae by Takhtajan (1997). They have been often been linked with Eucommiaceae, for which see Garryales (asterid I/lamiid).
[Papaveraceae [[Circaeasteraceae + Lardizabalaceae] [Menispermaceae [Berberidaceae + Ranunculaceae]]]]: vessel elements with simple perforation plates, in diagonal groups; with both vasicentric tracheids and nucleated libriform fibres; leaves (ternately compound or palmately lobed), secondary venation palmate; flower parts whorled ["K" opp. "C" opp. A] (not), "C" development always retarded; outer A nectariferous, whether petal-like or not; stigma wet [level?].
Age. Magallón et al. (2013) estimated an age of around (404-)394.3-389.9(-382) Ma for this clade, but it is much younger in most other scenarios; N. Zhang et al. (2012) estimate an age of slightly under 100 Ma, about 112.9 Ma is the age in Magallón et al. (2015), (146-)117(-102) Ma in J. Li et al. (2018: see other dates), ca 110 Ma in Y. Li et al. (2020: Ran-Pap) and (137.4-)133.5(-128.3) Ma (Peng et al. 2023a).
The time-of-origin of Papaveraceae, based ultimately on fossil data, is estimated to be ca 176.4 Ma, the oldest for any family included by Silvestri et al. (2021).
Evolution: Divergence & Distribution. Ranunculales, or perhaps more properly this node, contain ca 1.6% of eudicot diversity.
Endress (2011a) suggested that the presence of sepals and petals was a key innovation somewhere around here; optimization on the tree is not easy, and it is unclear at what level/for what purpose the sepals and petals of Papaveraceae-Papaveroideae and Ranunculaceae-Ranunculoideae might be considered to be the "same" (see below). Petal development is retarded throughout the order (e.g. Y. Zhang et al. 2023: in Epimedium pubescens both the inner K and C are delayed) Damerval and Becker (2017) suggest that there may have been two successive duplications of the AP3 lineage, involved in stamen and petal formation, here, although there are only two paralagous families in Papaveraceae, three elsewhere.
Genes & Genomes. A genome duplication some 124.4 Ma is linked to this node (Landis et al. 2018); J. He et al. (2022) also thought that there might have been a duplication here, but he was not sure..
Chemistry, Morphology, etc.. Wink (2008) noted that the berberine bridge enzyme (BBE), involved in the synthesis of berberine and other distinctive alkaloids from this clade (Kutchan 1998: berberine is also found in some Rutaceae, etc.) was quite widely distributed in flowering plants. Another gene in this pathway, FAD-dependent (S)-tetrahydroprotoberberine oxidase (STOX), is at least scattered in Ranunculales, and the different forms are quite similar in their activities if with different substrate specificities (Gesell et al. 2011). STOX and BBE genes were members of different clades of FAD-dependent oxidases (Gesell et al. 2011). For features of wood anatomy common in this part of the clade, see Carlquist and Zona (1988); some may be higher-level apomorphies.
PAPAVERACEAE Jussieu, nom. cons. - Back to Ranunculales
Plant herbaceous, mycorrhizae 0; numerous alkaloids [inc. protopine], little oxalate accumulation [?level]; laticifers +, articulated or not, anastomosing or not; latex +; roots diarch [lateral roots 4-ranked]; uniseriate rays common; cork?; subepidermal collechyma in stem; nodes 1:1-5 - 3≤:3≤; petiole bundles arcuate; leaves soft, ± fleshy, quite often glaucous, lamina margins usu. ±spiny toothed, leaf base broad; inflorescence determinate, terminal; flowers 2-merous, parts whorled; P = K + C, fugaceous, C 4, decussate, outer pair lateral; anthers extrorse; pollen microechinate, oncus with islets and granules of endexine; nectary 0; G connate, 2, collateral, occluded by secretion, placentation parietal (protruding-diffuse), (carpels gaping apically), compitum +; ovules anatropous, (with zig-zag micropyle), inner integument (2-)3 cells across; antipodal cells endopolypoid; carpel dorsal vein not reaching style; capsule septicidal [= placenticidal], (with false [commissural] septum - ?level); seeds ?curved; endotesta with coarse fibrillar network, calcium oxalate crystals +, exotegmen fibrous, (mesotegmen fibrous, fibres crossing), endotegmen walls thickened; endosperm nuclear; x = 7 (?8), nuclear genome [1 C] (0.034-)1.165(-39.757) pg.
44[list]/1902 - four subfamilies below. Largely N. Temperate, also scattered in Africa, South America, etc..
Age. Anderson et al. (2005) suggested an age of ca 119-106 Ma for crown-group Papaveraceae and Peng et al. (2023a) an age of around (123.8-)120.8(-117.9) Ma.
1. Papaveroideae Eaton - Back to Ranunculales
Rhizome 0; (berberine + [isoquinoline alkaloid]); nodes also 1:1; lamina vernation variable, entire to lobed, colleters +; flowers large, K protective, often green, enclosing the bud, C crumpled in bud; A (4-)many, (in multiples of two or three); pollen (orbicules +); (placentation ± axile), stigmas often confluent, dry; ovules many/carpel, outer integument (2-)4-10 cells across, inner integument 2-4 cells across, parietal tissue 2-4 cells across, nucellar cap ca 3 cells across, hypostase +; antipodals also multinucleate; capsule erect, also with transverse dehiscence, (indehiscent, schizocarp); exotesta (with stomata), often with thickened outer walls, unlignified, (anticlinal walls sinuous), (mesotesta +, parenchymatous), (endotegmen not persistent); n = 5-10 (14, 19); non-RNase-based gametophytic incompatibility system present; duplication of PAPACYL gene.
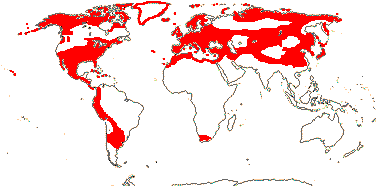
23/230 - three tribes below. Largely N. temperate. Map: from Ownbey (1958, 1961), Hultén and Fries (1986), Fl. N. Am. vol. III (1997), Fu and Hong (2000) and Malyschev and Peschkova (2004). [Photos - Collection (except Dicentra and Corydalis - see Fumarioideae).
Age. Crown-group Papaveroideae are ca 76.8 Ma (Y. Li et al. 2020: no 4) or (116.3-)101.2(-83.9) Ma (Peng et al. 2023a).
1A. Eschscholzieae Baillon —— Synonymy: Eschscholziaceae Seringe
Annual to perennial herbs (shrubs); (exudate +, watery); nodes 1:1(-3); hairs unicellular; subepidermal collenchyma in stem; hypanthium ± developed; pollen 4-11-colpate; capsule with 10 conspicuous longitudinal ridges, dehiscing explosively, opening from base; seeds arillate or not; exotesta with thickened inner and anticlinal cell walls; n = 6, 7, 11...
3/16: Eschscholzia (12). W. North America.
Age. Crown-group Eschscholzieae are estimated to be (78.0-)38.7(-10.3) Ma (Peng et al. 2023a).
[Papavereae + Chelidonieae]: G [?].
1B. Papavereae Dumortier (inc. Platystemoneae) —— Synonymy: Platystemonaceae Lilja
Annual to perennial herbs (small trees); (idioblasts/laticifers 0 - Platystemon); (uniseriate rays 0); (nodes also 1:1, 3:3); hairs multicellular and multiseriate (0 - Canbya); flowers (3-merous); A 6-many, (filaments expanded, toothed; pollen usu. tricolpate; G [3-24], style (styluli) +, (stigmatic lobes commissural); epistase, postament +; (megaspore mother cells several); fruits opening by valves/pores, carpel dorsal vein 0; n = 6-8, 11, 12, 14...
11/100-130: Meconopsis (79), Papaver (38), Argemone (32), Oreomecon (30). N. (warm) temperate, Argemone also South America (A. mexicana commonly introduced in the tropics), and southern Africa and the Cape Verde Islands (1 sp. in each - Papaver).
Age. The clade [Chelidonieae + Eschscholzieae] is ca 47.9 Ma (Y. Li et al. 2020).
1C. Chelidonieae Dumortier —— Synonymy: Chelidoniaceae Martynov
Annual to perennial herbs; latex orange, yellow or red; deltaacetylornithin +; nodes 3-5(-9):3-5(-9); hairs multicellular and terminally uniseriate (0); bracts foliaceous; (C 0 - Macleaya, Bocconia); pollen >3 pantoporate/colpate; G [(several)], (gynophore + - B.); ovules (1/gynoecium, basal - B.), both integuments 2-3 cells across [Coreanomecon]; fruit elongated, (not), (replum +); seeds arillate / strophiolate (not - Dicranostigma, Glaucium); exotesta tanniniferous, (mesotesta +), endotesta palisade, network layer of cellulose fibrils [?all], exotegmen fibrous-cuboid cells; n = 5, 6, 9, 10...
9/48: Glaucium (23), Bocconia (10). East Asia in particular, also eastern North America, Europe, Central and South America, West Indies).
Age. Crown-group Chelidonieae are ca 47.9 Ma (J. Li et al. 2018).
[Pteridophylloideae [Hypecoöideae + Fumarioideae]]: exudate watery; acetylornithine, (berberin) +; K 2, small, ± C-like, deciduous, median; A 4, 6 [≡ 8 sporangia]; pollen exine spinose; style long, ?colour, etc.; ovules with outer integument 2-4 cells across, inner integument ca 2 cells across, parietal tissue ca 4 cells across, nucellar cap 0 [?always], micropyle endostomal; exotesta palisade or not; (embryo long).
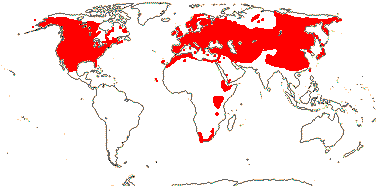
19/530. Mostly N. temperate, also S. Africa. Map: from Hultén and Lidén (1986), Fries (1986), Hong (1993), Fl. N. America vol. 3 (1997), Fu and Hong (2000), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003); Malyschev and Peschkova (2004) and Serban Procheŝ (pers. comm. S. Africa).
Age. Bell et al. (2010) offered an age for this node of (106-)88, 82(-61) My and (129-)123, 110(-104) Ma is the age suggested by Wikström et al. (2001: note topology) and (120.9-)112.0(-97.6) Ma (Peng et al. 2023a).
Leaf fossils of Potomacapnos about 120 Ma are placed in Papaveraceae in morphological phylogenetic analyses (Jud & Hickey 2013).
2. Pteridophylloideae Murbeck (Pteridophylleae) - Pteridophyllum racemosum Siebold & Zuccarini —— Synonymy: Pteridophyllaceae Nakai
Perennial herbs; alkaloids +; idioblasts 0, laticifers 0; leaf blade deeply pinnately lobed; inflorescence scapose, axis indeterminate, with cymose clusters of 1-4 flowers; A 4, alternating with C; pollen (2-, 6-colpate), orbicules +; style green, branches commissural [opposite inner C], short; ovules 1 (2)/carpel, epitropous, micropyle endostomal, raphe short, massive; fruit dehiscent; seed ± straight, aril 0; endotesta lacking crystals, tegmen thin; suspensor?, embryo small, cotyledons inconspicuous; n = 9.
1/1. Japan (Honshu).
[Hypecoöideae + Fumarioideae]]: exudate +, watery, often in non-articulated sacs; acetylornithine, (berberin) +; leaves to 3X ternately/palmately compound/deeply lobed; flowers transversely disymmetric; K small, C 4, decussate, heteromorphic, inner C 3-lobed, central lobe ± elaborated, holds pollen; nectary +, at abaxial base of dithecal A; A 6, opposite C, diadelphous; secondary pollen presentation +; pollen exine spinose; ovules ± campylotropous, parietal tissue ca 4 cells across, nucellar cap 0 [?always], funicle short; exotesta palisade or not, endotesta with cellulose fibrillar network, exotegmen not fibrous; (embryo long); x = 8.
19/530. Mostly N. temperate, also S. Africa. Map: from Hultén and Lidén (1986), Fries (1986), Hong (1993), Fl. N. America vol. 3 (1997), Fu and Hong (2000), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003); Malyschev and Peschkova (2004) and Serban Procheŝ (pers. comm. S. Africa).
Age. The age of this clade is estimated to be 106.3-95.5 Ma by Pérez-Gutiérrez et al. (2015a) and (113.1)98.7(-81.0) Ma by Peng et al. (2023a).
3. Hypecoöideae Prantl & Kündig - Hypecoum L. - —— Synonymy: Hypecoaceae Willkomm & Lange
Annual herbs; protopine alkaloids alone; outer C often 3-lobed, inner C lateral lobes large; A 4, dithecal, 2 median A with 2 vascular bundles [?= 2 unithecal A], lateral A with 1 vascular bundle, filaments alate; pollen bicolpate, deposited on inner C; style with linear branches, branches commissural; ovules several/carpel, inner integument ca 3 cells across; fruit various, schizocarpic/a lomentum, replum +/capsule; seeds not arillate; exotesta ± collapsing, transitory, endotestal crystals large rectangular, tegmen sclerified; suspensor of two massive cells; n = (7) 8, 9; cotyledons long, narrow-cylindrical.
1/18. The Mediterranean to W. China.
Age. The age of crown-group Hypecoöideae is 95.5-44 Ma (Pérez-Gutiérrez et al. 2015a) or (74.6-)51.2(-27.8) Ma (Peng et al. 2023a).
4. Fumarioideae Eaton
Annual or perennial herbs, (tuberous), (rhizomatous); deltaacetylornithin +; inflorescence terminal; K minute, (vascular trace 0), two outer C ± spurred, inner C apically coherent, with median joint [pollen container]; A 6, in two groups of three [diadelphous], central anther dithecal and lateral anthers monothecal, filaments of each triplet connate below anthers; endothecium from outer secondary parietal cell layer, inner layer dividing; pollen 3-12-colpate/6-12 syncolpate/6(-8) porate, (apertures with fluffy plugs [= ?tapetal endoplasmic reticulum]), surface smooth to verrucate, not perforated, (granulate infratectum); (placentation axile), style green, persistent, stigmas ± connate and laterally flattened, margin ± irregular, ?pollen presentation on margin, [?papillae 4]; ovules 1-many/carpel; fruit replum [persistent placental strands + [?how common]; seeds arillate/w. elaiosome; exotesta usu. pigmented, (palisade, crystaliferous), endotesta not crystaliferous; suspensor cells like a small bunch of grapes, (embryo undifferentiated); n = (6-)8(+); plastid transmission biparental, inversion of 11 genes (ndhB-trnR-ACG) in the IR region.
19/630. Eurasia, North America, North and South Africa, mountains of E. Africa. Photo: Corydalis Flower, another, Dicentra Flower.
Age. The age of crown-group Fumarioideae may be ca 74 Ma (Pérez-Gutiérrez et al. 2015a), (67.6-)65.7(-63.7) Ma (X. Xu et al. 2022) or (90.7-)77.5(-64.2) Ma (Peng et al. 2023b).
4A. Lamprocapneae H.-W. Peng, Liu & Wang - Lamprocapnos spectabilis (L.) Fukuhara
Perennial erect herbs; flowers pink, pendent, broadly cordate, outer C reflexed; outer wall of exotesta with inner part pigmented.
1/1. Siberia, northern China, Korea, and Japan.
[Ehrendorferieae [Dicentreae [Ichtyoselmideae [Adlumieae [Capnoideae [Dactylicapneae [Corydaleae + Fumariieae]]]]]]]: seed exotestal.4B. Ehrendorferieae H.-W. Peng, Liu & Wang - Ehrendorferia Fukuhara & Lidén
Perennial erect herbs; flowers yellow, erect; outer petals reflexed; stamens dorsally winged; fruit erect; elaiosomes 0; endotesta cells flat.
1/2. California, Baja California.
[Dicentreae [Ichtyoselmideae [Adlumieae [Capnoideae [Dactylicapneae [Corydaleae + Fumariieae]]]]]]: endotesta not crystalliferous.4C. Dicentreae Horaninow - Dicentra de Candolle
. Perennial herbs; inflorescence scapose; flowers pink or white tipped yellow, pendent, outer C reflexed; (filaments of each triplet coherent along their whole length - D. cucullaria); capsule elongated; endotesta cells loosely arranged; (cotyledon 1).
1/8. N.E. Asia inc. the Himalayas and West China, also the Pacific Northwest, the eastern U.S.A..
[Ichtyoselmideae [Adlumieae [Capnoideae [Dactylicapneae [Corydaleae + Fumariieae]]]]]: endotestal cells compressed.4D. Ichtyoselmideae H.-W. Peng, Liu & Wang - Ichtyoselmis macrantha (Oliver) Lidén
Perennial erect herbs; flowers yellow, pendent; K long, thin, outer C subspreading; stigma large and fiddle-shaped; placentae broad and flat.
1/1. N. Burma, S. China
[Adlumieae [Capnoideae [Dactylicapneae [Corydaleae + Fumariieae]]]]: plants annuals; filaments of each triplet completely fused.4E. Adlumieae H.-W. Peng, Liu & Wang - Adlumia de Candolle
Also biennials, scandent herbs; inflorescence axillary; flowers pink, pendent, very spongy; nectaries 0; elaiosomes 0.
1/2. East Asia, eastern North America.
[Capnoideae [Dactylicapneae [Corydaleae + Fumariieae]]]: replum covered by valve-margins.4F. Capnoideae H.-W. Peng, Liu & Wang - Capnoides sempervirens (L.) Borkhausen
Also biennial herbs; inflorescence terminal, cymose; flowers monosymmetric, spur 1; C pink, tipped yellow; fruit linear, erect.
1/1. Temperate North America, to the S.E. U.S.A..
[Dactylicapneae [Corydaleae + Fumariieae]]: inflorescence indeterminate.4G. Dactylicapneae H.-W. Peng, Liu & Wang - Dactylicapnos de Candolle
Also perennial herbs, vine; flowers yellow to orange (often reddish), pendent; nectaries conspicuous; stigma ± square; fruit elongated.
1/12. Himalayas to W. China.
[Corydaleae + Fumariieae]: bracteoles 0; flowers monosymmetric, 900 resupinate; spur 1, adaxial.Age. This node is (54.5-)44.1(-33.8) Ma (Peng et al. 2023b).
4H. Corydaleae Dumortier - Corydalis de Candolle —— Synonymy: Corydalaceae Vest, nom. illeg.
Perennial (annual) herbs, with taproot, often with root tubers; growth monopodial (sympodial); inflorescence racemose; bracts scarious; flowers yellow, spur short, saccate-quite long; stigma 4-10< papillate, (papillae double [on either side of stigma]), fruiting pedicels recurved; fruits not linear; (cotyledon 1 - taxa with tubers).
1/528 (estimates are 400-530 spp., for the latter number, see e.g. Peng et al. 2023b). North temperate, esp. the Himalayas and China, mountains of tropical E. Africa.
Age. Crown-group Corydaleae are estimated to be (51.0-)49.1(-47.2 Ma (X. Xu et al. 2022) or (52.1-)42.4(-32.4) Ma (Peng et al. 2023b).
4I. Fumariieae Dumortier —— Fumariaceae Marquis, nom. cons.
Also perennials, (climbers with tendrils) [modified leaves/leaflets]; pollen with more than three apertures, pantoporate, etc.; style caducous, articulated (not Ceratocapnos, Discocapnos), chlorophyll-less, stigma not flattened (yes), (stigma with membranous crest), stigmatic papillae 2 (not Fumaria); fruit a nut / few- (to 13- - Pseudofumaria) seeded capsule; elaiosomes 0 (Ps. +).
9/: Fumaria (50-55). Mediterranean, Central Asia to Himalayas, and E Africa.
Age. Crown-group Fumariieae are ca 33.2 Ma (see Pérez-Gutiérrez et al. 2015) or (44.0-)32.9(-23.4) Ma (Peng et al. 2023b).
Lamprocapnos around 65.62 MaEvolution: Divergence & Distribution. The ancestral habitat of Ranunculaceae may have been closed and humid conditions, the family beginning to diversify ca 120 Ma in early angiosperm forests in eastern Asia and spreading via the Bering land bridge to North America in the mid- to upper Cretaceous (Peng et al. 2023a). Groups like Ehrendorferia (Corydaloideae) and Hypecooideae moved into subhumid semiarid conditions, but diversification in these clades seems to have slowed down (Peng et al. 2023a); most dispersal events are associated with vicariance (or vice versa), but understanding diversification in the family depended on whether the j parameter was included and the number of areas into which the northern hemisphere was divided. Within Fumarioideae about three quarters of the species are from the Sino-Himalayan region, and Peng et al. (2023b) and Y. Y. Liu et al. (2024) discuss their diversification. The former group noted that diversification in Corydaleae and Fumariinae was rapid, but in Fumariinae in particular it was later (only within the last ca 15 Ma) and seemed to be associated with the prior adoption of the annual habit and the ability to grow in dry conditions, both of which could be thought of as preaptions, a pattern not observed in Corydaleae where diversification started much earlier, ca 42 Ma (Peng et al. 2023b). On the other hand, Y. Y. Liu (2024) suggested that divergence in Corydalis began in the Eocene ca Ma, but diversification in the Himalaya-Hengduan was nearly all after the Middle Miocene ca Ma.
Hoot et al. (2015: e.g. Table 3, Figs 3, 4) usefully optimised numerous characters on a phylogeny of the family; note the topology used (Pteridophyllum sister to the rest) and their interpretation of some of the characters. Thus the character "nectary +, at abaxial base of dithecal A" is placed as a synapomorphy for [Hypecoideae + Fumarioideae], Hoot et al. (2015) call nectaries in this position "staminodial nectaries"; see also Carrive et al. (2020) for nectaries. D. Kong et al. (2024) suggested that the flower of Pteridophyllum racemosum was very much like the "ancestral flower" (ibid., p. 7) of Papaveraceae as a whole. Kadereit et al. (2011) looked at evolution within Papavereae, offering some dates, while J. Li et al. (2018) focus on Bocconia and Macleaya (Chelidonieae). Pérez-Gutiérrez et al. (2015a) discuss the evolution of Fumarioideae-Fumarieae in particular, giving numerous dates. Gleissberg and Kadereit (1999) described the complexities of leaf development and interpreted them in a phylogenetic context: Leaflets may develop acropetally, basipetally, or both acro- and basipetally, depending on the species (Gleissberg 1998, see also 2004). Peng et al. (2024) carried out a joint-morphological-molecular analysis of the family, and they discussed the optimization of the 77 morphological characters that they examined in the context of a total evidence tree. They suggested polarities for many of these characters (ibid.: Fig. 5, App. 2), and many of their suggestions have been taken up; Ryberg (1960) has also been an invaluable source of data.
Ecology & Physiology. Several species of Fumaria and its relatives are chasmophytes. They grow in the apparently most inhospitable habitats from North Africa and the Mediterranean region eastwards despite their delicate and rather succulent habit (see also Pérez-Gutiérrez et al. 2012, 2015a). Dactylicapnos, Cysticapnos, and some other genera in Fumariieae are climbers with tendrils that represent modified leaves/leaflets (Sousa-Baena et al. 2018a).
In some species of Papaver stem stomata were found to be blocked by wax deposits, foliar stomata not so (Wulff 1898).
Pollination Biology & Seed Dispersal. The stigma of Corydalis,Fumaria and relatives, on which the pollen is deposited during the course of secondary pollen presentation, can be complex (Ryberg 1960; Brückner 1984). There is also secondary pollen presentation in Hypecoum; the pollen is deposited on the central lobe of the inner petals, and there it may be involved in both self and cross pollination (Dahl 1989; also S. Yang et al. 2019: H. erectum); Lv et al. (2024) talk about anther mimicry here. Lv et al. (2024) also suggest that pollen is deposited in a similar position in the Fumarioideae that they examined in what they call a "pollen container", while a "nectar holder" develops at the base of the outer petals, and is the spur in the characterizations above (El Ottra et al. 2023 suggest that 21 genera of Papaveraceae have secondary pollination presentation).
Papaveraceae - Papaver rhoeas, at least - have a fast-acting gametophytic self-incompatibility system which is very different from those in core eudicots (Franklin-Tong & Franklin 2003; Charlesworth et al. 2005; Franklin-Tong 2008b); L. Wang et al. (2019) describe the details of how the incompatible pollen dies by programmed cell death as the cell contents are acidified. Wheeler et al. (2009) suggest that the PrpS gene encoding the pollen S determinant lacks any homologues in other angiosperms that have similar incompatibility systems. The gametophytic self-incompatibility system of Papaver is (unusually) associated with a dry stigma (Wheeler et al. 2001; Allen & Hiscock 2008).
Quite a number of taxa, both forest herbs and chasmophytes and from both subfamilies, are myrmecochorous, the ants being attracted to the arils developed on the seeds (Fukuhara 1999; Lengyel et al. 2009, 2010); these arils have probably evolved several times.
Plant-Animal Interactions. Species of most of the subgenera of Parnassius butterflies (Papilionidae-Parnassiinae-Parnassiini), but not subgenus Parnassius itself, have caterpillars that eat Fumarioideae, and they are particularly diverse in eastern Asia (Michel et al. 2008; Simonsen et al. 2011; Condamine et al. 2012, 2018). Their movement on to this group was accompanied by an uptick in diversification, although it quite soon slowed down (Allio et al. 2020/2021).
Ceutorhynch seed weevils are quite commonly found on Papaveraceae; the weevils have moved on to the family perhaps only once, but there have been movements on to other hosts (Letsch et al. 2018).
Genes & Genomes. There is a genome duplication event associated with Papaveraceae and dated to ca 113 Ma (Landis et al. 2018). Y. Liu et al. (2021) thought that the PASOβ (Papaver somniferumβ) duplication occured ca 117.3 Ma and "predated the divergence of Ranunculaceae and Papaveraceae", yet they placed it on stem Papaveraceae. In any event, P. somniferum shows evidence of having two genome duplications (Liu et al. 2021).
The small single copy area of the plastome in Lamprocapnos spectabilis (Fumarioideae) is much reduced in size, in 2018 being the second smallest known in any plastome (the SSC is also small in Hoya and relatives (Apocynaceae) and it may even be absent in some Geraniaceae, q.v.). All in all, there have been quite extensive changes in the plastome of Lamprocapnos, IR boundary shifts - the IR has doubled in size - and inversions in the IR leading the way (Park et al. 2018). Increased substitution rates occur in some genes, and the rps15 gene is non-functional in the plastid, but there is a functional copy in the nucleus. Corydalis, too, shows extensive plastome variation with overlapping inversions, etc. (X. Xu & Wang 2021; Xu et al. 2022). Indeed, there is extensive variation in Fumarioideae and Hypecooideae (and also in Papaveroideae-Eomecon, sister to Sanguinaria, minute SSC, etc.) in genome size (see especially IR expansion, numbers of repeats) and gene content and arrangement - in terms of gene content in the species studied C. triternatifolia, but not other members of the genus studied, is remarkable (J. Cao et al. 2024). Variation in the plastome of Corydalis correlated quite well with the phylogeny (Xu et al. 2022).
Economic Importance. For Papaver - opium, etc. - see Bernáth (1998), and for oils from Papaver, see papers in Vollmann and Rajcan (2009).
Papaveraceae include a disproportionate number of notably serious and widespread weeds (Daehler 1997).
Chemistry, Morphology, etc.. 1-benzyltetrahydroisoquinoline alkaloids are found only here and in a small group of related genera of Rutaceae-Rutoideae, and in Apiaceae and Asteraceae (Kubitzki et al. 2011). Acetylornithine, reported from [Hypecoöideae + Fumarioideae] and Chelidonioideae (Lidén 1993), is involved in nitrogen transport (Jensen 1995). R. Zhang et al. (2020) survey alkaloids found in Fumaria. Opium, from which morphine was first isolated over 200 years ago, is produced by Papaver somniferum, and for its synthesis there in which gene duplication may have been involved, see Y. Li et al. (2020). Hartley and Harris (1981) found that the single species in each subfamily examined had distinctive UV fluorescence of unlignified cell walls. For nodal anatomy in the family, which varies considerably both within an individual and between species, see e.g. Ezalrab and Dormer (1963).
For the unusual (transverse) plane of floral monosymmetry in [Hypecoöideae + Fumarioideae], see e.g. Troll (1957), Ronse Decraene and Smets (1992a), Endress (1999), etc.; asymmetry of expression of the CYC gene is in the transverse plane here, and is rather late (Damerval et al. 2013; Hileman 2014). CYCLOIDEA genes have been duplicated in Papaveraceae s.l., and this may be connected with the development of monosymmetry (Kölsch & Gleissberg 2006; Damerval et al. 2007; see Jabbour et al. 2014 for analogous happenings in Ranunculaceae). In Corydalis and some other genera only a single outer petal is spurred and the flower is monosymmetric; there is a 90° rotation of the flower rather late in development so the spur is in the adaxial position (Ronse Decraene & Smets 1992a) and the monosymmetry is functionally vertical. There is a correlation between flowers with monosymmetry and indeterminate inflorescences, a variant on the correlation of determinate inflorescences and polysymmetric flowers.
The vascularization of the petals of Papaveroideae varies, but even if there is more than a single trace entering the base of the petals, the traces seem to have a single point of origin (Dickson 1935). I am unsure if all Papaveroideae have extrorse anthers, but anthers are clearly extrorse in other members of the family (Murbeck 1912). As in Ranunculaceae, the numerous stamens in Papaver, etc., may be derived from a paucistemonous condition. The nature of the androecium of Fumarieae in particular has occasioned much discussion, and it has sometimes been suggested that two anthers have each split into two, monothecal units, so there would be only four stamens altogether, but it is likely that the androecium consists of two dithecal and four monothecal stamens, the dithecal stamens being opposite the outer petals and the monothecal stamens being on either side of the insertion of the inner petals (e.g. Brückner 1992; Damerval et al. 2013). In Hypecoum the monothecal stamens have fused in pairs, hence the double vascular supply to two of what appear to be ordinary dithecal stamens, and in fumarioids the second stamen whorl may develop outside the first stamen whorl (Ronse Decraene & Smets 1992a for literature). The androecium of Pteridophyllum has also been interpreted as being derived from a flower with six stamens, the lateral stamens having been lost (Ronse Decraene & Smets 1992a); the stamens alternate with the petals and are diagonally arranged. D. Kong et al. (2024) provide floral diagrams for the four subfamilies. All in all, things are a little confused, and a careful study of androeccial development of taxa in this group will be very useful.
The "fluffy plugs" over the pollen apertures in many Fumarioideae are 3- or 5-lamellate structures that may be derived from tapetal endoplasmic reticulum (Pérez-Gutiérrez et al. 2015b, q.v. for other white line/lamellated structures, etc.). Interestingly, nectary development is associated with the expression of CRABSCLAW genes, unlike the development of nectaries in monocots and Ranunculaceae, but like that in Pentapetalae (Damerval et al. 2013: Proteales?).
When there are four carpels (mostly Papaveroideae-Papavereae) they are diagonally arranged (Ronse Decraene & Smets 1997b); see Brückner (2000) for discussion of carpel numbers in [Hypecoöideae + Fumarioideae]. Papaveraceae are described as having hollow styles, although the central space may become occluded by papillae (Hanf 1935). The ovary of Fumaria may have only a single ovule and the fruit is then nut-like and indehiscent. Jernstedt and Clark (1979) describe stomata in the exotesta in some Papaveroideae.
For general information, see Ryberg (1960: Fumariaceae), J. W. Kadereit (1993: as Papaveraceae), Lidén (1993: as Fumariaceae and Pteridophyllaceae), Lidén (1986: Hypecoöideae + Fumarioideae), also see Dahl (1989: Hypecoum, general), Tebbitt et al. (2008: Corydaloideae) and Grey-Wilson (2014: Meconopsis et al.), also Hegnauer (1969, 1990) and Preininger (1986), both chemistry, Hao et al (2018: chemistry and medecine), Vent and Mory (1973: alkaloids in Chelidonieae), Carlquist et al. (1994: wood anatomy). Léger (1895: vegetative morphology and anatomy), Mikhailova (2015: rootstock variation in Corydalis, Bersillon (1955: nodal anatomy and floral vasculature), Bull-Hereñu and Claßen-Bockhoff (2011b: Fumarioideae inflorescences), Murbeck (1912: floral morphology), Mair (1973: monosymmetry in Fumaria), Erbar (2014) and Zhang and Zhao (2018), both nectaries, Tarasevich (2014) and Pérez-Gutiérrez et al. (2015b), both pollen, Ronse Decraene and Smets (1990a: comparison with Begoniaceae), Becker et al. (2005: Eschscholzia) and Zumajo-Cardon et al. (2017: Bocconia), all floral development, Dickson (1935: floral vascularization), Ernst (1967: floral morphology, Platystemoneae), Gonnermann (1980: Papaveroideae gynoecium), Kadereit and Erbar (2011: style morphology and development), G. Dahlgren (1981: stigma secretions), Brückner (1984: stigma and carpel, 1992: Pseudofumaria, 1993 and references, all carpels, Fumarioideae), Goebel (1932) and Endress (2011b), both ovule orientation, Guignard (1903: Hypecoum), Sachar (1955), Sachar and Mohan Ram (1958), and Berg (1968), all embryology, Ahn et al. (2023: Coreanomecon), Brückner (1982: fruit, mostly Papaveroideae), 1983 (seed, mostly Papaveroideae), Gunn (1980: seeds - c.f. generic limits), Fukuhara and Lidén (1995: testa anatomy) and Röder (1958), Kapil et al. (1980), Ghimire (2019) and particularly Meunier (1891) for seed coat anatomy and development and arils. Additional information on Pteridophyllum is taken from Pérez-Gutiérrez et al. (2016: pollen) and Brückner (1985: fruit and seed); the seeds have a cellulose network in the endotesta like that of some Papaveroideae.
Phylogeny. There have been some uncertainties in the phylogeny. Pteridophyllum is rather distinctive (although included in Fumariaceae by Cronquist 1981) with its rather harsh deeply pinnately-lobed and fern-like leaves; in versions 8 and earlier of this site it was placed as a monotypic subfamily sister to the rest of Papaveraceae, and that is where Hoot et al. (2015) had found it to be, although support was not strong. Pteridophyllum linked with [Hypecoöideae + Fumarioideae] in molecular analyses, although without much support for any particular position, but in total evidence analyses there was strong bootstrap and somewhat less strong posterior probability support for a sister group relationship with Hypecoum in particular (W. Wang et al. 2009). Pérez-Gutiérrez et al. (2015a) and Sauquet et al. (2015: matk sequence suspect) all found its position to be unclear, but Peng et al. (2023a: plastome analyses, 2024) found quite strong support for a position sister to [Hypecoöideae + Fumarioideae]. See also Judd et al. (1994) and Nikolic (1995) for earlier studies. For a recent phylogeny of the whole family, see Peng et al (24) - they looked at 97 species (this includes all genera), using 2 nuclear and 7 plastome markers, also 77 morphological characters; support values were mostly high.
Papaveroideae-Chelidonieae are discussed by J. Li et al. (2018), who found that [Sanguinaria + Eomecon] were sister to the rest of the tribe (see also Peng et al. 2023a: strong support), however, in their work on the relationships of Coreanomecon, Ghimire et al. (2019) placed [Dicranostigma + Glaucium] in that position. Stylophorum and Dicranostigma were found to be paraphyletic by Peng et al. (2023a). Relationships in Papavereae: Papaver was early found to be paraphyletic and Meconopsis polyphyletic (Kadereit & Sytsma 1992; Kadereit et al. 1997, 2011; Carolan et al. 2006); see Xiao and Simpson (2015, 2017) for Meconopsis in particular. In the chloroplast tree of Peng et al. (2023a) Platystemoneae (= [Meconella [Platystemon + Hesperomecon]]) were well embedded in Papavereae, and with strong support.
For a phylogeny of Fumarioideae, see Lidén et al. (1997), Pérez-Gutiérrez et al. (2015a), Sauquet et al. (2015). Dicentra has been dismembered, and Dicentra (now = Lamprocapnos) spectabilis has turned out to be sister to all other Fumarioideae, and the old Corydaleae becomes highly paraphyletic, relationships being something like [Lamprocapnos [Ehrendorfia [Dicentra [Icthyoselmis [Adlumia [Dactylicapnos + The Rest]]]]]] (Pérez-Gutiérrez et al. 2015a; Hoot et al. 2015; Peng et al. 2023a). However, morphological studies have tended to recover a Fumarieae and Corydaleae; for relationships within the former, which is monophyletic, see Pérez-Gutiérrez et al. (2012), Hoot et al. (2015), etc.. Peng et al. (2023b: nuclear ITS and six plastid regions, 159 spp.) looked at Fumarioideae in some detail, general relationships previously proposed were confirmed. Focussing on Corydalis, X. Xu et al. (2022: 39 spp., plastomes) found quite good support for a topology in which groupings were rather different for the earlier infrageneric classification that relied on rootstock variation; the clade [C. adunca + C. stricta] was sister to the rest of the genus. However, J.-T. Chen et al. (2023: 226 spp., 65 plastid genes, 152 low-copy nuclear genes) found some conflict between plastome and nuclear analyses. Y. Y. Liu et al. (2024: 1 spp, 95 plastome and 8 single-copy nuclear genes, over 3¼ nuclear SNPs)
Classification. A.P.G. II (2003) allowed as an option the possibility of including Papaveraceae, Fumariaceae, and Pteridophyllaceae in an expanded Papaveraceae, and the limits of Papaveraceae were later formally expanded (e.g. A.P.G. III 2009).
The generic groupings above are mostly taken from Hoot et al. (1997), Kadereit et al. (1994, 1995) and especially from W. Wang et al. (2009). I had been conservative in placing Pteridophyllum; Hoot et al. (2015) placed it in a separate subfamily, and this does seem reasonable, although its position in Hoot et al. was sister to the rest of the family. Within Papaveroideae, generic limits needed major adjustments (e.g. Kadereit & Baldwin 2011; Kadereit et al. 2011, 2015; Kadereit 2017). It seems that Platystemoneae will have to be included in Papavereae (Xiao & Simpson 2017). Ghimire et al. (2019) suggest genera to be included in Chelidonieae. Within Fumarioideae, Dicentra has been dismembered, being paraphyletic (Lidén et al. 1997, etc.), and the morphology of the old Dicentra is the basic morphology of Fumarioideae as a whole. Any reclassification of this subfamily is going to entail the pulverisation of the Corydaleae, indeed, if Corydaleae (= Corydalis) and Fumarieae are recognized, then an additional seven tribes will be needed for an exhaustive classification... (see the tree in Peng et al. 2023b). Indeed, these tribes were all given names by Peng et al. (2024), and outside Papaveroideae apart from Corydaleae all the tribes, etc., have only a single genus, and four have but a single species.
For an infrageneric classification of Meconopsis, see Xiao and Simpson (2017). J.-T. Chen et al. (2023) reclassify Corydalis, recognising 4 subgenera and 39 sections, about half of which are new or newly circumscribed.
Previous Relationships. In some earlier systems, Papaveraceae s.l. were grouped with Brassicaceae, etc., in Parietales, a single-character group characterised by having parietal placentation. Hardly surprisingly, its members are now scattered throughout the tree.
[[Circaeasteraceae + Lardizabalaceae] [Menispermaceae [Berberidaceae + Ranunculaceae]]]: vascular rays broad; flowers often 3-merous, K, C and A opposite each other, K/P ± petal-like, "C" +, nectariferous [= A], development notably retarded; AP3 gene triplicated.
Age. Bell et al. (2010) suggested an age for this node of (106-)92, 85(-71) Ma; ages of (126-)120, 111(-105) Ma were suggested by Wikström et al. (2001), about 98.2 Ma by Magallón et al. (2015), (127.3-)126.4(-125.6) Ma by W. Wang et al. (2016a), ca 108.1 Ma by J. Li et al. (2018) and (116.4-)107.2(-96.3) Ma by Y. Sun et al. (2020: note topology).
Evolution: Ecology & Physiology. Lardizabalaceae and Menispermaceae are both lianes, sometimes vines, and they both have very large sieve tube plastids. Fossil woods of lianes that can be identified as belonging somewhere in this part of the tree are relatively common in woods Cretaceous-Palaeogene age; woods of Vitaceae-Vitoideae are first known from the Palaeogene (S. Y. Smith et al. 2013a).
Pollination Biology & Seed Dispersal. If the evolution of nectaries/nectariferous petals can be placed at this node, details of the pattern of expresssion of AP3-III petal identity genes become interesting (see also Sharma et al. 2011). Nectariferous petals are often interpreted as being staminodial in origin (Erbar 2014 and references), but that idea has been contested (Sharma et al. 2014).
Genes & Genomes. The duplication of Cycloidea genes can be pegged to this node (Jabbour et al. 2014); they are involved in the development of monosymmetric flowers in Ranunculaceae (see below).
Chemistry, Morphology, etc.. For the vasculature of the sepals/outer tepals, see Hiepko (1965); for their development, see Zhang et al. (2009 and literature). When the "petals" are modified stamens, as seems to be the case here, they are delayed in development (L. Zhao et al. 2016b). For pollen morphology, see Nowicke and Skvarla (1982). For chromosome size, see Langlet (1928, 1931) and Okada and Tamura (1979).
[Circaeasteraceae + Lardizabalaceae]: leaves palmately compound; K/P with a single trace; anthers extrorse; endosperm cellular.
Age. Anderson et al. (2005) suggested an age of ca 116-107 Ma for this node, Bell et al. (2010) ages of (102-)87, 81(-66) Ma, Wikström et al. (2001) ages of (121-)115, 106(-100) Ma, Magallón et al. (2015) an age of about 86.3 Ma, J. Li et al. (2018) an age of ca 91.3 Ma, and J. He et al. (2022) an age of 101.5 Ma, while at ca 126.1 Ma, the estimate of Tank et al. (2015: Table S2) is the oldest.
The time-of-origin of Lardizabalaceae, based ultimately on fossil data, is estimated to be ca 156.7 Ma (Silvestri et al. 2021).
CIRCAEASTERACEAE Hutchinson, nom. cons. - Back to Ranunculales
Herbs; chemistry?; cork cambium?; true tracheids?; nodes 1:1; petiole bundle ?arcuate; lamina margin toothed, venation largely dichotomous; inflorescence terminal, cymose or thyrsoid, or flowers terminal, perfect or not; flower parts spirally arranged; A (1-)2-6(-8), not obviously opposite P; pollen exine layered-striate; G 1-9, compitum ?0; ovules ± apical, unitegmic; embryo sac tetrasporic, 4- or 8-nucleate; fruit an achene; seed coat degenerating, thin; embryo relatively large; x = 7 (?8, ?6), plastome with ca 49 kb and ca 3.5 kb inversions in the LSC region.
2 [list]/2 - two genera below. N. India to S.W. and W. China. Map: from Fu and Hong (2000).
Age. Anderson et al. (2005) suggested an age of ca 84-72 Ma for the divergence of these genera, Bell et al. (2010) ages of (65-)48, 45(-30) Ma; ages in Wikström et al. (2001) are (72-)68, 54(-50) Ma, in Y. Sun et al. (2020) (73.6-)51.8(-31.0) Ma, and inJ. he et al. (2022) they are ca 49.8 Ma.
1. Circaeaster agrestis Maximowicz
?Prophyll; leaves simple; bracteoles 0; P +, uniseriate, small, ± green, ± K-like, 2-3; A latrorse?, anthers bisporangiate, monothecal; stigma ± sessile; ovule straight, pendulous, integument 2-3 cells across, parietal tissue 0; embryo sac Adoxa type; fertilization mesogamous [pollen tube entering ovule laterally, penetrating integument]; achene with uncinate hairs; endosperm with chalazal haustorium; n = 15; plastome infA a pseudogene, accD 0.
1/1. India (Himalayas), W. China. Photo: Circaeaster Habit.
Age. Fossil fruits from the Potomac-Puddledock formation, Early to Middle Albian and perhaps 107-104 Ma, have been compared with those of Circaeaster. The two are indeed in general rather similar, although the stigma is relatively broad and sessile in the fossil, however, the pollen, which is rather consistently associated with the stigma, is monocolpate and with a low-verrucate surface, and so quite unlike that of Circaeaster (Crane et al. 1994). Given the pollen differences, relationships of the fossil around here seem unlikely.
2. Kingdonia uniflora Balfour f. & W. W. Smith —— Synonymy: Kingdoniaceae Airy-Shaw
Annual; prophyll adaxial; leaves two-ranked, palmatisect; K 5(-7); 8-13 clavate glands [?staminodes]; G 3-9; stylulus short; ovules hemianatropous, integument 2-5 cells across, parietal tissue ca 2 cells across, early disorganizing, integument short, nucellus naked; endosperm helobial; n = 9, chromosomes "Ranunculus type"; plastome with all but 2 ndh genes as pseudogenes or 0.
1/1. W. and N.W. China.
Evolution: Divergence & Distribution. Y. Sun et al. (2020) noted the over-representation of gene families that were involved in DNA repair but under-representation of genes responding to stress - and the loss of most of the plastome ndh genes - might be consistent with the latter loss. Indeed, Kingdonia appears to live in a stable, overall quite equable and stress-free environment, but it reproduces largely vegetatively, hence perhaps the DNA repair genes... (Sun et al. 2020).
Carrive et al. (2020) suggested that there had been a transition to spiral perianth phyllotaxis in this clade.
Pollination Biology. Heterodichogamy, etc., in Kingdonia is discussed by X.-M. Wang et al. (2012).
Genes & Genomes. Y. Sun et al. (2020) sequenced the genome of Kingdonia and found that it was very large (1004.7 Mb), probably because there has been a recent genome duplication and long terminal repeats are abundant.
Sun et al. (2017) found substantial changes in the organization of the plastome of Circaeasteraceae, including two inversions in the large single copy of both genera. There were also some gene losses/pseudogenisations, most notably in all but two of the ndh genes in Kingdonia; although such changes are quite common in mycoheterotrophs and parasites, they are uncommon elsewhere (Sun et al. 2017).
Chemistry, Morphology, etc.. Kingdonia may have up to four bundles departing from the single foliar trace (shades of a 1:2 node?) and, like Circaeaster, several root hair zones on the roots (Foster & Arnott 1960; Ren & Hu 1998). Xylem perforation plates may also be scalariform. Kingdonia appears to have an adaxial prophyll (see s.e.m. of axillary buds in Ren et al. 2004 - no comment made).
Circaeasteraceae do not show the same relationship between the stamens and perianth members of many other Ranunculales. The floral formula of Kingdonia is very variable, although P5 A11 staminodes)+ 5 G6 is common, and the perianth members have a single trifid vein, indeed, all floral organs are innervated by a single vein, apart from the first perianth member, which has two traces (as in some Ranunculaceae, see Ren et al. 2004; Ning et al. 2023). The genus also has 8-13 glistening clavate glands immediately inside the perianth whorl; these are described as petals by Tamura (1993) and as staminodes by Ren et al. (2004) and Ning et al. (2023) and may secrete nectar. Mesogamy, i.e. the pollen tube entering the ovule laterally by penetrating the integument, is reported for Circaeaster, and the mature endosperm is differentiated into two zones; Circaeaster also has endosperm with a chalazal haustorium (see Junell 1931).
General information is taken from Tamura (1993: Kingdonia, in Ranunculaceae) and C.-Y. Wu and Kubitzki (1993: as Circaeasteraceae); see also Nowicke and Skvarla (1981) for pollen, Tobe (1995, as Ranunculaceae) for embryology, Hu et al. (1990), Ren and Hu (1995) and Tian et al. (2006) for information on Circaeaster agrestis, and Mu (1983) and Ren et al. (1998, 2004) for information on Kingdonia uniflora. The inside cover of Act. Bot. Bor.-Occid. Sinica 24(1) (2004) has a photograph of K. uniflora flowers with excellent details of gross morphology.
Classification. Keeping Kingdoniaceae separate from Circaeasteraceae was optional in A.P.G. II (2003).
Previous Relationships. Kingdonia has been placed in Ranunculaceae-Anemoneae, e.g. by Kosuge et al. (1989). The dichotomous venation of the leaves and the separate carpels of Circaeasteraceae have attracted attention as possibly indicating a very "primitive" group.
LARDIZABALACEAE R. Brown, nom. cons. - Back to Ranunculales
Lianes; benzylisoquinoline alkaloids 0; (plant Al-accumulators); nodes 3:3; petiole bundles arcuate; plant glabrous or hairs uniseriate; buds perulate; leaflet vernation conduplicate, margins entire; inflorescence axillary, racemose; flowers six-merous; "C" small, apices nectariferous; staminate flowers: A 6, connective often prolonged apically; tapetal cells 2-nucleate; pollen exine smooth; carpelate flowers: staminodia +; G 3, also spiral, placentation marginal, carpels with postgenital fusion and secretion, stigma wet; suprastylar extragynoecial compitum; ovules campylotropous, inner integument 2-4 cells across; fruit a berrylet; germination phanerocotylar; x = 8 (?11, ?7), chromosomes "small", nuclear genome [1 C] (0.031-)1.259(-50.422) pg.
7[list]/40 - two groups below. South East Asia and Chile. Map: see Taylor B. (1967) and Ying et al.() 1993).
Age. Anderson et al. (2005) suggested an age of 95-66 Ma for crown-group Lardizabalaceae, Bell et al. (2010) ages of (51-)38, 35(-23) Ma, Wikström et al. (2001) ages of (88-)81, 76(-67) Ma and W. Wang et al. (2020) an age of (109.3-)76.9(-46.0) Ma.
Kajanthus lusitanica was described from Portugese Cretaceous deposits around 113 Ma and the characters that can be taken from it are identical to those of Sinofranchetia, so it may even be assignable to crown-group Lardizabalaceae (Mendes et al. 2014). Indeed, in their angiosperm-wide analysis Schönenberger et al. (2020) found several maximum parsimony positions for this fossil in Lardizabalaceae and Berberidaceae (see also López-Martínez et al. 2023a: Table 3 - consistently in Ranunculales alone).
1. Sargentodoxoideae Thorne & Reveal - Sargentodoxa cuneata - (Oliver) Rehder & E. H. Wilson —— Synonymy: Sargentodoxaceae Hutchinson
Triterpenoid saponins 0; cork cambium deep-seated; tanniniferous cells +; leaves ternate; plant dioecious (some flowers perfect); carpelate flowers: K 4-9, C 5-7; staminodes +, like inner T; G 40<, stipitate; ovule 1(2)/carpel, ± campyltropous, pendulous, outer integument 4-5 cells across; receptacle becoming fleshy; surface of testa featureless; endosperm reserve?; n = 11.
1/1. China.
2. Lardizabaloideae Kosteletzky —— Synonymy: Decaisneaceae Loconte, Sinofranchetiaceae Doweld
(Shrubs); oleanone triterpenoid saponins +; (vessel elements with scalariform perforation plates); (stomata cyclocytic); leaves (odd-pinnately compound - Decaisnea [D.]), petiolules long (terminal leaflet only), (leaflets with basal tooth or lobe), (secondary veins pinnate); plant monoecious (dioecious), (flowers perfect): (outer T 3), ("C" 0); staminate flowers: (A 3, 8), filaments connate (not); tapetal cells (to 4 nucleate), (amoeboid - Stauntonia); (pollen grains colporoidate), (tricellular); carpelate flowers: staminodes +; G 3-12, (placentation laminar), (stigma peltate); ovules many/carpel (few),n(hemitropous, anatropous), (micropyle endostomal - D. [D.]), outer integument 2-4(5-6 - D.) cells across, parietal tissue 3-8 cells across, (nucellar cap ca 2 cells across); (antipodal cells persistent - D.); (fruit a fleshy follicle), placenta fleshy in fruit; testa multiplicative, exotestal cells lignified, elongated, ± oblong [D.] or unlignified, fibrous [Akebia, Hoelboellia], hypodermal cells thickened; endosperm starchy [D.] or with hemicellulose, (nuclear - D.); n = 14-16, ?17, 18.
6/39: Stauntonia (28). South East Asia and Chile (Lardizabala, Boquila). [Photos - Lardizibala Staminate flower, Boquila Flowers, Fruit, Fruit close-up.]
Age. Wikström et al. (2001: Decaisnea sister to rest) suggested ages of (69-)61, 51(-42) Ma for crown-group Lardizabaloideae.
Kajanthus lusitanicus, from Late Aptian to Early Albian deposits in Portugal, is associated with Akebia quinata by López-Martínez et al. (2023a: Fig. 2C; see also Mendes et al. 2014), but other positions in Ranunculales are also possible.
Evolution: Divergence & Distribution. W. Wang et al. (2020) offer ages for a number of nodes in the family, which perhaps originated in East Asia. They discuss the biogeography of the family, i.a. suggesting that it arrived in South America via long distance dispersal.
Ecology & Physiology. The Chilean Boquila trifoliata is reported to mimic the leaves of a variety of species on which it climbs, the one stem mimicking different species sequentially, and even mimicking the plant closest to it when climbing on a different species; herbivory may be reduced (Gianoli & Carrasco-Urra 2014). See also Alseuosmia (Asterales-Alseuosmiaceae) and Loranthaceae. Incroyable!
Pollination Biology. Smets (1986) suggested that the nectaries are staminal nectaries; stamen and petal develop primordia develop immediately adjacent to each other in Holboellia (X.-H. Zhang & Ren 2011). In Decaisnea nectar may be secreted by stamens, while in Akebia the stigmatic secretions are sweet (Endress 1995; Erbar 2014 and references), but nectar production and pollination are poorly known here. In some taxa, at least, the stigmatic exudate spreads and joins adjacent stigmas so forming a hyperstigma (Wu and Kubitzki 1993; X.-H. Zhang & Ren 2011).
Chemistry, Morphology, etc.. Wood fluorescence? The leaves of Akebia pentaphylla, at least, are peltately palmate (Kim et al. 2003).
X.-H. Zhang and Ren (2011) depict dehiscence of the staminodes of Decaisnea insignis; the pollen looks normal - but are there some kind of viscin strands? Nowicke and Skvarla (1982) studied the pollen morphology especially of Sargentodoxa; there may be additional apomorphies for that genus. The seeds of Akebia, at least, are embedded in some kind of fleshy tissue.
For additional general information, see Wu and Kubitzki (1993), Qin (1997), and Christenhusz (2012) and other papers in Bot. Mag. 29(3). 2012; for chemistry, see Hegnauer (1966, 1989, also 1973, as Sargentodoxaceae) and Zheng and Yang (2001), nodes (Dormer 1954), seed surface, Xia and Peng (1989), carpel development, van Heel (1983), ovule morphology (X.-h. Zhang et al. 2015), and some anatomy, Yong and Su (1993); X.-H. Zhang et al. (2005, 2009, 2012) provide detailed studies of Sinofranchetia.
Phylogeny. Sargentodoxa is sister to the rest of the family (Hoot et al. 1995b, see also Hoot 1995a; Kofuji et al. 1994). Decaisnea may be sister to the remainder (Kofuji et al. 1994; Hoot et al. 2015); it has a number of distinctive (apomorphic) embryological features (H. F. Wang et al. 2009b). However, based on the recent discovery of the fossil Kajanthus, very similar to Sinofranchetia, Mendes et al. (2014) suggest that the root may be misplaced, Sargentodoxa being nested within the crown group. In an analysis of chloroplast data, F. Wen et al. (2021) found that Akebia was polyphyletic.
Classification. Although Sargentodoxa has a number of autapomorphies (see above, also X.-H. Zhang & Ren 2008), there is no compelling reason to segregate it as a family (H.-F. Wang et al. 2009a).
See Christenhusz (2012) for a summary of the family.
[Menispermaceae [Berberidaceae + Ranunculaceae]]: (berberine + [isoquinoline alkaloid]); nucellar cap +; endosperm nuclear.
Age. The age of this node may be (119-)113, 103(-97) Ma (Wikström et al. 2001); on the other hand, Magallón et al. (2013) estimate an age of around 65.9 Ma, Magallón et al. (2015) an age of ca 89.9 Ma, Anderson et al. (2005) an age of 116-105 Ma, Bell et al. (2010) an age of (99-)83, 77(-63) Ma, Jacques et al. (2011) an age of 125-115.6 Ma, J. Li et al. (2018) an age of ca 103 Ma, J. He et al. (2022) an age of ca 97.7 Ma, while at some 127.9 Ma and (127.1-)126.2(-125.3) the estimates of Tank et al. (2015: Table S2) and W. Wang et al. (2016a) respectively are the oldest.
Evolution: Divergence & Distribution. Diversification may have increased at this node (98.2-)93.8(-89.9 Ma (Magallön et al. 2018).
Evolution: Divergence & Distribution. There have been nested diversification rate increases here (98.2-)93.8(-89.9) Ma and again ca 10 Ma later (Magallón et al. 2018).
MENISPERMACEAE Jussieu, nom. cons. - Back to Ranunculales
Lianes (vines), stem twining, (shrubs, trees); also/or aporphine alkaloids, sesqui- and diterpenoids +, (plant tanniniferous); successive cambia frequent; (rays narrow); secretory cells +, in files; sclereids common; crystals common; nodes 3:3; stomata various, often ± cyclocytic; hairs unicellular to uniseriate; leaves simple, lamina ± peltate [at least with the base joining the top of the petiole], margins entire (toothed; lobed), petiole pulvinate at base and apex; plants dioecious; inflorescence axillary; flowers small, parts whorled or spiral; K with a single trace, (1-)6(-12), "C" 0-8, often connate, ± petal-like and nectariferous, (clasping A); staminate flowers: A 3, 6, 12 (1-40, if many, not all opposite petals), anther thecae horizontal, (bisporangiate, monothecal); pollen tricolporate, endapertures circular; pistillodes +/0; carpelate flowers: staminodes +/0; G (1-)3(-30<), with postgenital fusion and secretion, opposite P [Cissampelos], five bundles per carpel, gynophore common, style terminal, stigma ± flaring; suprastylar extragynoecial compitum +; ovules 2/carpel, often unitegmic, hemianatropous, micropyle endostomal (zig-zag), integuments folded, outer integument 2-5 cells across, inner integument 2-3 cells across, parietal tissue 2-11 cells across, chalazal part large to massive; antipodals multiplying, multinucleate; fruit a drupelet, 1-seeded, endocarp dorsoventrally curved, (with longitudinal ridging); seed with condyle [placental intrusion], curved, coat undistinguished (exotesta tabular, lignified); endosperm + (0), variously ruminate (smooth), embryo long, cotyledons incumbent, longer than the radicle; n = (9-)11-13(+), n = 13 (?14. ?12), chromosomes small, nuclear genome [1 C] (0.06-)1.762(-52.099) pg.
71/442: [list] - nine groups below. Pantropical, usually lowland (map: see Wickens 1976; Frankenberg & Klaus 1980; van Balgooy 1993; Fu & Hong 2000; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; Malyschev & Peschkova 2004; Rosa Ortiz-Gentry, pers. comm. 2004; Australia's Virtual Herbarium i.2013). [Photo - Fruit, Fruit.]
Age. Anderson et al. (2005) thought that crown-group Menispermaceae were ca 80-70 Ma, Wikström et al. (2001) gave a rather younger age of (59-)53, 48(-42) Ma, while that of Bell et al. (2010) at (52-)35, 33(-18) Ma is even younger. However, Jacques et al. (2011: see other estimates) estimated an age of 124.4-103.3 Ma, and W. Wang et al. (2012: fossil calibrations) suggested ages of (115.2-)109.1, 106.3(-101.7) My; (54.2-)41.5(-32) Ma and ca 110 Ma are the ages recently suggested by W. Wang et al. (2016a: focus on Ranunculaceae) and (2016b) respectively. Take your pick.
Callicrypta, from the mid-Cretaceous of Siberia, has very small flowers (carpelate) with the parts more or less opposite, or forming spirals, and may be Menispermaceae; however, it is unclear what a link between Menispermaceae and Amborellaceae - hardly close - that the fossil is supposed to represent might look like (c.f. Krassilov & Goloneva 2004). Fossils menisperms are reported from the Upper Turonian of ca 89.3 Ma from the Czech republic and many fossils are known from Lower Ypresian deposits of ca 55.2 Ma age (Jacques et al. 2011; see also Jacques 2009a). Although Cretaceous records of Menispermaceae seemed questionable to Herrera et al. (2011), W. Wang et al. (2012) accepted that of Prototinomiscium vangerowii, from the Turonian of the Czech Republic (Knobloch & Mai 1986; see also Anderson et al. 2005).
1. Chasmantheroideae Luersson
(G several); ?ovules; seed subglobose-reniform; endocarp bilaterally curved; condyle ± boat-shaped or a ventral groove; endosperm ruminate, embryo spathuliform, cotyledons foliaceous, divaricate.
28/145. Pantropical, Atlantic North America.
Age. Chasmantheroideae are (115.8-)101(-87) Ma (Jacques et al. 2011) or ca 107 Ma (W. Wang et al. 2016c).
1A. Coscinieae J. D. Hooker & Thompson
Sepals in three whorls; staminate flower: A 6 or more, anthers with transverse dehiscence, filaments ± connate/synandrial; carpelate flower: C 0; drupelets subglobose, style remnant subapical-adaxial; endocarp ?compression.
3/6. Eastern Asia, temperate to tropical.
Age. Coscinieae are (64.4-)36.9(-14.1) Ma (Jacques et al. 2011: Calycocarpum - diverged ca 75.7 Ma - sister).
1B. Burasaieae Endlicher
(Lamina compound - Burasaia), staminate flower: A free/connate/(synandrium), 6 (more, fewer); (pollen also tricolpate, triporate); ovules anatropous, (single integument, 3-6 cells across - Tinospora); endocarp straight, dorsiventrally compressed, (condyle 0); seeds boat-shaped.
25/139: Tinospora (36), Odontocarya (36). Pantropical, east Asia.
Age. Crown-group Burasaieae are (105.1-)90.8(-77.8) ma (Jacques et al. 2011: Calycocarpum sister to Coscinieae) or (109-)99.5(-88) Ma (W. Wang et al. 2016c).
2. Menispermoideae Arnott
Staminate flowers: (anthers connate, extrorse); (pistillode 0); carpelate flowers: (staminodes 0); (G 1), style lateral to basal; endocarp curved, ± laterally compressed, often with transverse ridging as well; cotyledons strap-like/rounded, fleshy, accumbent (incumbent).
44/300. Pantropical, east North America, eastern Asia
2A. Menispermeae de Candolle
A 6<, free; pollen tricolpate; (pistillate flowers with staminodes; stone semiannular-crescentic; cotyledons strap-like.
2/3. East North America, eastern Asia.
Age. Menispermeae are ca 31.1 Ma (Jacques et al. 2011).
Fossil fruits named Palaeosinomenium hengduanensis from Upper Eocene deposits (36-)35(-34) Ma of S.W. China were compared with the extant Sinomenium acutum; Palaeosinomenium itself is known from widely distributed Middle Eocene (and older) deposits in the northern hemisphere (M. Wu et al. 2022).
[Anomospermeae [Limacieae [Tiliacoreae [Pachygoneae [Spirospermeae + Cissampelidae]]]]]: A 6.
Age. This clade is (109-)95.8(-80.5) Ma (Jacques et al. 2011: note topology - Diploclisia excluded, sister to whole lot, if included (114.3-)99.5(-84.8 Ma)).
2B. Anomospermeae Miers —— Synonymy: Pseliaceae Rafinesque
(Localized pericyclic ectopic cambia - Abuta); A 6 (3), free/(connate)/(synandrium); (endosperm not ruminate), cotyledons strap-like.
13/80: Abuta (31). South America, East Asia to Australia, the Pacific.
Age. Crown-group Anomospermeae are estimated to be (75.7-)66.2(-57.4) My or (93-)76.3(-60.7) Ma (Lian et al. 2019 and Jacques et al. 2011 - both with other similar estimates).
[Limacieae [Tiliacoreae [Pachygoneae [Spirospermeae + Cissampelidae]]]]: ?
Age. The age of this clade is around (101.7-)87(-71.4) Ma (Jacques et al. 2011).
2C. Limacieae Prantl - Limacium Loureiro
K in three whorls; A 6, free; endocarp with longitudinal ridge, laterally weakly convex.
1/2. Temperate and tropical East Asia.
[Tiliacoreae [Pachygoneae [Spirospermeae + Cissampelidae]]]: cotyledonary area subcylindric.
Age. This clade is some (89.1-)74.4(-62.1) Ma (Jacques et al. 2011).
2D. Tiliacoreae Miers
A >6/6/<6, free/connate/synandrial
18/111: Tiliacora (22), Abertisia (19), Sciadotenia (19). ±Tropical, Pacific Islands (Map: Lian et al. 2023: Fig. 1).
Age. Crown-group Tiliacoreae are around (65.1-)48.5(-32.2) Ma (Jacques et al. 2011), (61.3-)49.4, 49.3(-34.9) Ma (W. Wang et al. 2012) or (63.2-)48.0(-33.2) Ma (Lian et al. 2023).
[Pachygoneae [Spirospermeae + Cissampelidae]]: endocarp longitudinally and transversely ribbed.
Age. This clade is (72-)63.3, 62.1(-52.6) Ma (W. Wang et al. 2012).
2E. Pachygoneae Miers
staminate flowers: K 6, C 6; A 6; pistillode +; carpelate flowers: K 6, C 6; A 6, free, staminodes +; stigma/style linear.
4/45: Hyperbaena (22). Pantropical.
Age. Crown-group Pachygoneae are around (75.3-)64.6(-56.4) Ma (Jacques et al. 2011: n.b., including a well-embedded Spirospermeae, not including Haematocarpus) or (60.1-)48.7,47.1(-33.5) Ma (W. Wang et al. 2012: with Haematocarpus, + ca 5 Ma).
[Spirospermeae + Cissampelidae]: filaments connate.
Age. This clade is (69.8-)60.2, 59.7(-50.2) Ma (W. Wang et al. 2012).
2F. Spirospermeae R. Ortiz & W. Wang
Tree; staminate inflorescence with cymules; A 6 or more, connate; drupelets stipitate.
4/10. Madagascar (west tropical Africa).
2G. Cissampelideae J. D. Hooker & Thompson
Staminate flowers: K 6, C 3; A >6, synandrial; pistillode 0; carpelate flowers: monosymmetric; K 1, C 2; A synandrous, staminodes 0; pollen tricolpate/-porate; (tapetum amoeboid); G 1, stigma flaring ["crest-like"]; cotyledons shorter than the radicle, cotyledonary area subflattened.
5/128: Stephania (69}, Cyclea (32). Pantropical, Pacific Islands.
Age. Cissampelideae are around (68.8-)53.4(-37.9) Ma (Jacques et al. 2011).
Fossil endocarps of Stephania are reported from early Palaeocene deposits ca 64 Ma in Argentinia (Jud et al. 2018).
Evolution: Divergence & Distribution. Major clades within the family diverged during the late Cretaceous (Jacques et al. 2011: Table 5 for dates, Menispermeae sister to rest). Indeed, extensive diversification and migration in the family, perhaps Laurasian in origin, may have occurred around the K/T boundary during a period spanning (82.2-)71.7, 60.3(-45.3) Ma (W. Wang et al. 2012), and New World clades are embedded in Old World clades (Ortiz et al. 2016). W. Wang et al. (2016c) and Lian et al. (2020) give dates for various clades within Burasaieae and Lian et al. (2023) for those in Tiliacoreae.
South American is proving to be quite diverse in fossil menisperms. Doria et al. (2008) found Eocene leaf fossils from northern Colombia, and well preserved endocarps have been recorded from two Palaeocene localities in Colombia, one dated to ca 60 Ma (Herrera et al. 2011). Some have been identified as Stephania, now known only from the Old World, but also in Palaeocene deposits from North America (Han et al. 2017) and southern South America (Jud et al. 2018). If the identifications are correct, the younger ages for crown-group Menispermaceae above are incorrect. For the menisperm fossil record, see also Jacques et al. (2007).
Relationships within Tiliacoreae have a strong geographical component: [Neotropical [African + Indo-Austronesian]], and there were two dispersal events from the Afrotropics to Indo-Malesia and three from Indo-Malesia the Australasia (Lian et al. 2023).
Wefferling et al. (2013) and Ortiz et al. (2007, esp. 2016) discuss character distributions of fruit and seed and their optimization on the tree; polarization of the variation is not so easy. Hoot et al. (2009) optimized characters on a tree with Menispermum and immediate relatives (Menispermeae) sister to the rest of the family. Jacques and Zhou (2010) used Procrustes analyses to understand variation in endocarp morphology; they placed this in the context of a molecular tree.
Ecology. Menispermaceae are an important component of the climbing vegetation in the tropics, perhaps especially in the New World (Gentry 1991).
Plant-Animal Interactions. Larvae of the large noctuid moths of the subfamily Catocalinae use Menispermaceae as their major food source throughout the tropics, although they can also be found on other plants like Erythrina (some Menispermeae have pentacyclic Erythrina-type alkaloids). The adult moths, with their saw-like proboscides, attack ripe or ripening fruits and cause a considerable amount of damage to commercial crops (Fay 1996).
Economic Importance. The muscle relaxant D-tubocuranine is obtained from Chondrodendrum tomentosum. This is also a major ingredient of the South American poison curare and is put on arrows and darts.
For the basic floral arrangement of Menispermum, at least, see e.g. Eichler (1875) and Walch and Blaise (2022a); the adaxial member of the outer whorl is no. 2 to be initiated. Flowers can be monosymmetric, as in the carpelate flowers of Stephania dielsiana, where there are 1 + 2 sepals and petals and a single carpel (H. Wang et al. 2006; Meng et al. 2012); the staminate flowers are always polysymmetric. Tepals in e.g. Menispermum canadense have only a single trace (Smith 1928). Q.-j. Wang et al. (2018) examined nectar secretion on the "petals" of Stephania; there is variation here, including whether or not the nectariferous tissue (= nectarioles) is asociated with sieve tubes. There is considerable variation in pollen morphology in the family (Harley & Ferguson 1982 and references) which needs to be integrated with the clades that are becoming evident. The upper of the two ovules is epitropous and fertile, the lower is apotropous (Mauritzon 1936; Joshi 1939). Joshi (1939) suggested that in the unitegmic Tinospora cordifolia, the thinner upper part of the integument represented the outer integument, the thicker part, both integuments fused. There is apparently a period of 6-8 weeks between fertilization and first division of the zygote in T. cordifolia (Sastri 1964).
Additional general information is taken from Réaubourg (1906), Kessler (1993), and Jacques (2006); Hegnauer (1969, 1990) summarized information on chemistry, Wilkinson (1986) described leaf anatomy and Jacques and de Franceschi (2007), wood anatomy; see H. C. Wang et al. (2006) for floral development, H.-Y. Zhang et al. (2022) for androecial variation and Harley (1985 and references) for pollen morphology. Much work has recently been carried out on the complex drupelets of the family; see also Jacques (2009b), Jacques and Zhou (2010), and Ortiz (2012: curved embryos develop in different ways).
Phylogeny. Hoot et al. (2009: three chloroplast genes) had found that Menispermum and Sinomenium formed a clade sister to all the rest of the family in two gene analyses, but with little support (see also Ahmad et al. 2009; Jacques et al. 2011), although in three-gene analyses they were in a position like that found by Ortiz et al. (2007) where was sister to other Menispermoideae. Although Tinomiscium was strongly supported as sister to all other Menispermaceae (Ortiz et al. 2007), the sequences were corrupt (R. Ortiz, pers. comm.). The genus belongs in the [Tinosporeae (now in Burasaieae) + Coscinieae] clade, Tinosporoideae (= Chasmantheroideae), a clade that had at most moderate bootstrap support (Ortiz et al. 2007; see also W. Wang et al. 2009: three chloroplast and one nuclear genes, morphology, support weak, sampling poor; Ortiz 2012). The tree above follows relationships suggested by analyses of molecular data in Ortiz et al. (2016); much of the backbone and many of the relationships within the tribes have strong support. However, relationships within Tiliacoreae and Anomospermeae are less well supported, and the position of Pachygoneae was also poorly supported, indeed, when morphological data were added, Pachygoneae formed a clade with Tiliacoreae (Ortiz et al. 2016).
The monophyly of Chasmantheroideae was well supported in the analyses described by Wefferling et al. (2013), and the tropical Coscinieae are sister to the rest of the subfamily (W. Wang et al. 2012; Wefferling et al. 2013; Hoot et al. 2015). For some relationships in Burasaieae, see W. Wang et al. (2016c) and Lian et al. (2020).
Menispermoideae include the rest of the family and are well supported (but less supported in Wefferling et al. 2013 and Hoot et al. 2015). Within Menispermoideae the temperate Menispermum and relatives (Menispermeae) are sister to the other taxa, often with strong support, and there are other well supported relationships (Ortiz et al. 2007, 2016; W. Wang et al. 2012; Wefferling et al. 2013: c.f. Jacques et al. 2007: morphological data only, variously treated; Jacques & Bertolino 2008, some samples mislabelled, see Jacques et al. 2011). The old Menispermeae, Fibraureae and Peniantheae are polyphyletic (see also Wang et al. 2007a). Hoot et al. (2009: three chloroplast genes) had found that Menispermum and Sinomenium formed a clade sister to all the rest of the family in two gene analyses, but with little support (see also Ahmad et al. 2009; Jacques et al. 2011), although in three-gene analyses they were in a position like that found by Ortiz et al. (2007).
Lian et al. (2019) discuss relationships in Anomospermeae, with a focus on Neotropical taxa. Hong et al. (2001) discuss phylogenetic relationships in Menispermeae. Within Stephania, S. tetrandra may be sister to the rest of the genus (Xie et al. 2015). Tiliacoreae. For relationships here, see Lian et al. (2023: 7 plastid and 2 nuclear loci, sampling good); although Anisocycla turned out to be polyphyletic, Lian et al. dealt with the problem (two new genera).
Classification. For the tribal classification above, I follow Ortiz et al. (2016).
Thanks. To Rosa Ortiz, for continuing discussions and for information.
[Berberidaceae + Ranunculaceae]: perennial herbs, rhizomatous; vessel elements with simple perforation plates [lower in the tree?]; nodes also multilacunar; vascular bundles V-shaped, in herbaceous taxa often closed, not in a single ring [scattered or in concentric rings]; leaf base ± sheathing, (paired petiolar stipules +); inflorescence terminal; outer P with three or more vascular traces; nectar discharge by bursting cells; AP3-III gene expressed in P whorl alone; compitum 0; outer integument at least 4 cells thick; endosperm reserves other than oil or protein.
Age. Anderson et al. (2005) suggested an age of ca 104-90 Ma for this node, Bell et al. (2010) an age of (87-)72, 67(-54) Ma, Xue et al. (2012) an age of ca 77.8 or 89.1 Ma, Magallón et al. (2015) an age of about 80.3 Ma, and Wikström et al. (2001) an age of (106-)100, 84(-78) My; the ca 128 Ma in Z. Wu et al. (2014) and ca 127.9 and 124.4 Ma in Tank et al. (2015: Table S1, S2) are dramatically older (see also Yu & Chung 2017; (124.3-)123.7(-123.3) Ma is an age suggested by W. Wang et al. (2016a). A crown-goup age of ca 84.1 Ma is found in Jacques et al. (2011: note topology), ca 92.8 Ma in J. Li et al. (2018), (96.5-)80.0(-57.4) Ma by Y. Sun et al. (2020) and (97.8-)90.1(-81.2) Ma by J. He et al. (2022). However, note the ages of fossils perhaps attributable to Ranunculaceae...
Chemistry, Morphology, etc.. Podophyllotoxin, a cyclic lignan, is known from Podophyllum and its immediate relatives, and has recently been found in Helleborus (Z. Liu et al. 2020); it is scattered elsewhere and at least sometimes produced by endophytic fungi (Biswas et al. 2020).
Nowicke and Skvarla (1981) thought that aperture columellae might be a synapomorphy for [Berberidaceae + Ranunculaceae] (see also Nowicke & Skvarla 1979 for pollen). For the expression of the AP3-III gene, see Sharma et al. (2011).
For general information, see Janchen (1949).
BERBERIDACEAE Jussieu, nom. cons. - Back to Ranunculales
Myricetin, isoprenylated flavonoids +, tanniniferous; cork also pericyclic; hairs 0 (unicellular or -seriate); lamina vernation curved or conduplicate (complex in Podophyllum, etc.), margins variously toothed (entire), (secondary venation pinnate); inflorescence often racemose; flower trimerous, parts whorled; "C" 6; ("C"/A primordia +); A 6, opening acropetally by flaps; tapetal cells multinucleate; G 1, postgenital occlusion by secretion, stylulus short, stigma ± funnel-shaped, corrugated, dry or wet; ovule micropyle exostomal, outer integument 4-12 cells across, inner integument 2-5 cells across; antipodal cells endopolyploid, (not persistent); fruit a berrylet; exotestal cells lignified, oblong-fibrous to cuboid; endosperm with hemicellulose; embryo minute; x = 7 (?6); chromosomes large, nuclear genome [1 C] (0.274-)2.958(-31.887) pg.
14 [list: as subfamilies]/701 - three clades below. Mostly East Asia and E. North America, also South America, a few species general N. temperate, scattered in Africa. Map: from Ahrendt (1961), Hong (1993), Fl. N. Am. vol. 3 (1997), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Malyschev and Peschkova (2004). [Photos - Collection.]
Age. Anderson et al. (2005) suggested a crown-group age of ca 88-72 Ma for Berberidaceae, while the age in W. Wang et al. (2016a) is (124.3-)123.7(-123.3) m.y, estimates in Yu and Chung (2017), at a little under 100 Ma, are intermediate, and those in Y. Sun et al. (2018), at around 35 Ma, and Jacques et al. (2011), at ca 57 Ma, are (far) younger. Ca 77.2 Ma is the estimate in M. Guo et al. (2021: see relationships in order).
1. Podophylloideae Eaton —— Synonymy: Diphylleiaceae Schultz-Schultzenstein, Epimediaceae Menge, Leonticaceae Airy Shaw, Podophyllaceae Candolle, nom. cons., Ranzaniaceae Takhtajan
(Podophyllotoxin +); leaves palmately compound / 2-foliolate / simple and ± deeply lobed, (stipules +); lowermost branch of inflorescence subtended by reduced leaf; (flower single), (bracts, bracteoles 0 - Achlys - A.), (dimerous - Epimedium - E.); P (0 - A.), 4-18, "C" (0, 7-9), (4, with nectar spurs); A (sensitive), (4) (-19 - Podophyllum, A.), (dehiscence by slits), (extrorse - E.); microsporogenesis successive [?all], pollen striate (spiny), (diads; tetrads); (flower with cortical vascular system), G with horizontal/horizontal-oblique groove abaxial to stylulus, placentation parietal/basal, (stylulus long, stigma flat); ovules (1-5)-many/carpel, (hemitropous), micropyle (zig-zag), (integuments lobed), inner integument 2-3 cells across, (endothelium +), parietal tissue 0-2 cells across, (postament +); (megaspore mother cells several - Diphylleia); fruit also an achene, or follicle, (transverse groove in middle of carpel, dehiscence there); (seeds arillate); outer integument multiplicative [?all]; n = 6.
8/75: Epimedium (55). Mostly (Europe to) East Asia (some desert xerophytes) and W. or E. North America. [Photo - Podophyllum Flower © R. Kowal, Fruit, Ripe Fruit.]
Age. Crown-group Podophylloideae are thought to be (104.7-)81.2(-18.5) Ma (Yu & Chung 2017), 36-27 Ma (Y. Sun et al. 2018) or ca 74.4 Ma (M. Guo et al. 2021).
2. Nandinoideae Heintze —— Synonymy: Nandinaceae Horaninow
(Shrubby), (tuberous); cyanogenetic [Nandina - N.]; wood diffuse porous, rays broad, complex; nodes 5≤:5≤; leaves to 3x palmately compound, petiole concave at the base, stipules ?+; lowermost branch of inflorescence subtended by ± leaf-like inflorescence bract; (P ca 10, green, "C" 0 - N.); (nectary 0 - N./basal shallow sac on small "C" - G.); A-C common primordia; (A dehiscence by slits); ovules 2-5/carpel, funicle 0 and endothelium + [N.]; (fruit with pericarp evanescent or bladder-like); funicle swollen, (testa undifferentiated - G.), (testal cells thin-walled, endotegmic cells large, lignified - N.); n = 8, 10 [N.].
4/20: Gymnospermium (13). E. Europe to Japan.
Age. (97.5-)85.6(-69.2) Ma or (90.1-)63.8(-27.5) Ma is the crown-group age of this subfamily (W. Wang et al. 2016a and Yu and Chung 2017 respectively), while a mere 33-13 Ma is the estimate in Y. Sun et al. (2018) but ca 46.5 Ma in M. Guo et al. (2021).
3. Berberidoideae Kosteletzky
Shrubs, (leaves deciduous)/herbaceous, rhizomatous [Ranzania - R.]; berberastine [alkaloid] +; wood ring porous, (vessel elements with scalariform perforation plates - Berberis [B. s. str.]); cork cambium deep-seated [?level]; nodes 3:3, 5≤:5≤; (petiole bundles arcuate - B.> s. str.); (short shoots + - B. s. str.), leaves trifoliate/odd-pinnate/unifoliolate/reduced to (branched) spines [on long shoots - B. s. str.], margins often spiny-toothed, secondary veins various, stipules +; inflorescences borne on short shoots/not, (determinate); K 3-12, C-like, staminodes 6, ± petal-like, with paired basal nectaries; A 6, sensitive; tapetum amoeboid, cells 4-8-nucleate; microspore development successive, tetrads isobilateral, pollen 6-12 colpate, or apertures irregular (spiraperturate), wall undifferentiated; flower with cortical vascular system [R.]/0, placentation parietal/basal; ovules 1-many/carpel, parietal tissue ca 2 cells across; (megaspore mother cells several); endosperm cellular [Mahonia], embryo long; x = 7.
2/629: Berberis s.l. (628). General N. temperate, also South America, N. and E. Africa.
Age. Crown-group Berberidoideae are (87.1-)68(-51.7) Ma (Yu & Chung 2017) or 28-6 Ma (Y. Sun et al. 2018) - but see comments below about the topology of the phylogeny. Ca 46.8 Ma is the estimate in M. Guo et al. (2021).
Evolution: Divergence & Distribution. For additional dates in the family and a critical evaluation of the fossil record, see Yu and Chung (2017). Dates, etc., from Hsieh et al. (2020) are not included given the different topologies of trees based on plastome data (the topology they used) and other analyses.
Berberis is by far the most widely distributed genus in the family, and fossils have been reported from Palaeocene deposits ca 60 Ma old in northeast China; Oligocene and younger fossils are known from elsewhere in the Northern Hemisphere (Y.-L. Li et al. 2010). The genus may have originated in North America, with one move to South America and two to Eurasia (Adhikari et al. 2015). Relationships between the four major clades of Berberis s.l. (they have all been called genera) are unclear, but Hsieh et al. (2022) suggest that two of these clades may be the result of hybridisations between Berberis s.str. and Mahonia.
Several genera in Berberidaceae are disjunct and/or are found only in limited areas, and many of the taxa involved may have originated in East Asia. Despite the probably late Cretaceous age of the family, these disjunctions may be relatively recent, forming within the last 10 Ma, although disjunctions in the desert xerophytes Bongardia and Leontice are probably rather older (Donoghue & Smith 2004; W. Wang et al. 2007b). The crown-group age of the speciose Epimedium is estimated to be a mere (2.4-)2.1(-1.9) Ma, speciation perhaps being associated with the rise of the Qinghai-Tibet plateau (M. Guo et al. 2021). Y. Sun et al. (2018) estimated that 27 dispersals and three vicariance events were needed to explain the distributions of their fifteen terminals; Berberidaceae may have originated in eastern Asia.
Nandina and Caulophyllum look rather different, as do Berberis s.l. and Ranzania, but molecular analyses place members of the pairs close, indeed, the latter pair of genera are almost identical florally.
Y. Zhang et al. (2023: Table 1) summarize floral variation in the family (but not Berberidoideae). See M.-Y. Zhang et al. (2012) for pollen evolution.
Pollination Biology & Seed Dispersal. LeBuhn and Anderson (1994) describe how the sensitive stamens of Berberis thunbergii work, noting i.a. that they return to their resting position in about twenty minutes and that there are viscin threads (sic) in the pollen; details of the process vary between species (e.g. c.f. Sharma & Verma 2016), but little is known about pollination here.
In Epimedium the nectaries are inside spurs coming from the four inner tepals or in depressions on those tepals, with cell expansion being particularly important in spur development (Xie et al. 2022); see Erbar (2014) for the diversity of nectaries in the family (Vancouveria, sister to Epimedium, lacks spurs).
Seeds of a number of taxa, both forest herbs and desert xerophytes, have elaiosomes/are arillate (not necessarily different things) and are myrmecochorous (Lengyel et al. 2009, 2010). Berberidoideae in particular have berries - note that the berries of Nandina can kill cedar waxwings when the latter are binge-eating because they contain cyanogenic glycosides (Zona 2022).
Plant-Bacterial/Fungal Associations. Some seventy species of Berberis (inc. Mahonia) are alternate hosts for Puccinia graminis, the economically very important black stem rust of wheat and other grain crops in Poaceae-Pooideae.
Vegetative Variation. Leaf morphology, and the number and kinds of leaves produced per flush, varies considerably in Berberidaceae. Pabón-Mora and González [= P.-M. & G.] (2012) discuss leaf development in Berberis s.l. with a focus on what the spines on the long shoots of Berberis might represent. The spines are articulated, sometimes branched and stipulate, while there are simple and also articulated photosynthetic leaves (i.e. they are unifoliolate) on the short shoots. The scale leaves/cataphylls on the short shoots of Berberis are basically stipular (G. & P.-M. 2009), and these are prophyllar in position - interestingly, the first pair of leaves often seem to be borne more or less in the same plane as the cataphylls (P.-M. & G. 2012: e.g. Fig. 3C, F-M). Mahonia s. str. (with ca 100 species) hybridises with Berberis. It has compound foliage leaves on its long shoots, and although at the base there are almost spine-like cataphylls, these are clearly modified compound leaves (P.-M. & G. 2012: Fig. 8: E-J). They are quite clear about the stipules (ibid. pp. 479, 484): "The stipulate leaf base is present in all leaf homologs of Berberís s. str. that is, the spiny leaves of the long shoots, the folióse leaves of the short shoots, and the cataphylls." "Stipules are formed in the flanks of the leaf base in all leaf homologs of Berberís s. 1. (i.e. foliage leaves, spiny leaves, and cataphylls, except in the cataphylls of the flowering short shoots of Mahonia." However, the labelling of the figures leaves something to be desired; an asterisk on projections at the leaf base may be the stipules, but these are not shown on the basal cataphylls, which are presumably prophylls.
Genes & Genomes. It has been suggested that ancient hybridization between Berberis and Mahonia resulted in the taxa called Alloberberis and Moranothamnus (Hsieh et al. 2022).
For the evolution of the chloroplast genome, see Y. Sun et al. (2018; also Hsieh et al. 2022); there has been considerable expansion of the inverted repeat in Berberis s.l. in particular. Variation in the length of the accD gene was considerable; there have been deletions there, but also the insertion of a considerable number of 5aa-long repeats (Hsieh et al. 2022).
Chemistry, Morphology, etc.. The epidermal waxes of Podophyllum are solid rods.
For nectary morphology, see S. Su et al. (2021); they are common in the family, and apparently associated with the corolla. Although Podophyllum has many stamens, single stamens or groups of stamens are opposite the innermost perianth members (Schmidt 1928); L. Zhao et al. (2014) described all floral organs (except the carpel) as originating in whorls of three in the related Dysosma, and, as elsewhere in the family (Su et al. 2021), there were no C-A primordia (thus absent in Plagiorhegma and Epimedium pubescens see Y. Zhang et al. 2023). X. L. Liu et al. (2017) did record C-A primordia from Gymnospermium, although there they described much reduced staminodes with pollen sacs immediately adaxial to the bases of the filaments that were possibly "homologous" with nectariferous petals in Caulophyllum, and the six perianth members were not strictly decussating. Ghimire and Heo (2012) described the anther tapetum of Berberidoideae as being glandular; if true, multinucleate tapetal cells would still separate Berberidoideae from other Berberidaceae. Successive microsporogenesis has been reported (Min et al. 1995). The carpel in Berberidaceae varies in its orientation. According to Chapman (1925, c.f. e.g. Feng & Lu 1998), the gynoecium is derived from two or three carpels, with the gynoecia of the n = 6 clade alone being derived from two carpels (Kim & Jansen 1998), however, the gynoecium is probably unicarpelate throughout the family (Brückner 2000 for a summary). The single carpels of Dysosma (L. Zhao et al. 2016b) and of Plagiorhegma (Y. Zhang et al. 2023) are shown as being obliquely oriented. There seems to be some confusion over the nature of the micropyle; here I follow Zhang et al. (2023) and call it exostomal. Ghimire et al. (2010) described the thinly crassinucellate ovules of Gymnospermium (Podophylloideae) as having a well-developed endothelium, while in Nandina, aside from the endothelium, the outer epidermal cells of the inner integument are anticlinally elongated (Kumazawa 1938a). The carpel walls of Caulophyllum (Podophylloideae) do not surround the maturing blue seeds, so the plant is a kind of gymnosperm...
Some general information is taken from Schmidt (1928), Loconte (1993) and Stearn (2002: herbaceous Berberidaceae) and chemistry from Hegnauer (1964, 1989). Terabayashi carried out extensive studies on the floral anatomy, morphology, etc., of Berberidaceae, and he synthesised his findings in Terabayashi (1985a, b). For some wood anatomy, see Shen (1954: mostly Nandina) and Dulin and Kirchoff (2010: Mahonia), for nodal anatomy, see Dormer (1954), for inflorescence development in Berberis, see Bull-Hereñu and Claßen-Bockhoff (2011b), for floral development of Caulophyllum (common stamen-nectary primordia), see Brett and Posluszny (1982, also Remizowa 2019), for that of Gymnospermium see X. L. Liu et al. (2017) and for that of Epimedium and Plagiorhegma, see Y. Zhang et al. (2023), for the chaotic arrangement of the androecium in Achlys, see Endress (1989a; Tucker 1991), for pollen, see Nowicke and Skarvla (1981), for microsporogenesis, see Furness (2008b), for spore/gamete development in Diphylleia, see Huang et al. (2010), for the female gametophyte, see Huss (1906), for that of Podophyllum, see Sreenivasulu et al. (2010), and for arils, see Pfeiffer (1891).
Phylogeny. Nandina is a very distinctive plant, and in the past it has been segregated as a monotypic family or subfamily (as in versions 7 and earlier of this site). However, Nickol (1995) had suggested on morphological grounds (for the morphology of Nandina, see also Terabayashi 1983) that it was close to Caulophyllum, although it was placed sister to the rest of the family in the most parsimonious tree that he found. Early molecular studies (e.g. Adachi 1995) also found relationships between the two genera, and these have since been confirmed, as by Kim et al. (2004), Hoot et al. (2015), and Y. Sun et al. (2018), even if Nandina did sometimes tend to wander about the tree (e.g. Kim & Jansen 1996, 1998).
The three subfamilies above, which more or less form a tritomy, appear in the analyses carried out by Kim et al. (2004), for example; Podophylloideae have only moderate support (see also W. Wang et al. 2007b). W. Wang et al. (2009) confirmed these three main clades, and although molecular support for a [Nandinoideae + Berberidoideae] clade was weak, it was much strengthened in analyses that included morphological data; these relationships were also found in the plastome analyses of Y. Sun et al. (2018) and M. Guo et al. (2021). However, Hoot et al. (2015) found the relationships [Nandinoideae [Podophylloideae + Berberidoideae], but again support could have been stronger, while W. Wang et al. (2016a), Z.-D. Chen et al. (2016) and Yu and Chung (2017: weak support) found the relationships [Berberidoideae [Nandinoideae + Podophylloideae]], but things were switched around - [Nandinoideae [Berberidoideae + Podophylloideae]] - in BEAST analyses. Hsieh et al. (2022) looked at 93 plastomes from 80 species that were in all the 19 genera that they recognised, and they also looked at nuclear ribosomal genes. The relationships that they found, including those in Berberis s.l., differed in the analyses of the different genomic compartments. Using their tribal names and with the numbers of genera in each placed in parentheses, relationships from the plastome data were [[Jeffersonieae (2) [Epimedieae (2) [Bongardieae (1) [Achlydeae (1) + Podophylleae (4)]]]] [[Nandineae (1) + Leonticeae (3)] [Berberideae (4) + Razanieae (1)]]]; there have of course been various earlier hypotheses of relationships, especially in the Podophylleae area (see Hsieh et al. 2022). The three subfamilies are generally agreed upon, but their relationships are unclear; although the sampling in the Angiosperms353 analysis in the Seed Plant Tree of Life (i.2023) is very much poorer that that of Hsieh et al. (2022), i.a. Ranzania not being included, relationships in the former, quite well supported, are [Berberidoideae [Podophylloideae + Nandinoideae]], and in the latter [Podophylloideae [Berberidoideae + Nandinoideae]]. All this is why the three families are in a tritomy above.
For relationships in Epimedium, see Y. Zhang et al. (2014: 58 spp, amplified fragment length polymorphism data); they found that two species of subgenus Rhizophyllum were sister to the rest of the genus, but otherwise sections were either poorly supported or polyphyletic, although a number of species groupings were evident. M. Guo et al. (2021: plastid genomes, 32 spp.) found that E. koreanum, the only member of sect. Macroceras that they examined, was sister to sect. Diphyllon, but in the latter section relationships tended to be very poorly supported and the series were not monophyletic. E. diphyllum and E. koreanum were in a small polytomy immediately above subgenus Rhizophyllum in the analysis of Zhang et al. (2014).
Relationships within Berberis s.l. are [B. higginsiae [B. nivenii + The Rest]] (Adhikari et al. 2015, but see Yu & Chung 2017 for problems with the B. higginsiae sequences); these two species were in a clade sister to the rest in Yu and Chung (2017), while B. claireae, from Baja California, was sister to all other Berberis s. str. (but 4>% of that clade was sampled). The sampling level was similar in the plastome analysis of Hsieh et al. (2022).
Classification. For an infra-familial classification, see W. Wang et al. (2009). Hsieh et al. (2022) divided the family, which they thought had 19 genera, into three subfamilies (the same as the above) and 9 tribes. Berberis above includes Mahonia, and although Wu and Chung (2017) split this group into four genera, sampling of Berberis in particular was rather exiguous; Hsieh et al. (2022) follow suit.
Previous Relationships. Fruit dehiscence in some Berberidaceae and Papaveraceae is transverse, at least in part. Although on this account these families are similar (e.g. Endress 1995a), little else indicates immediate phylogenetic relationships.
RANUNCULACEAE Jussieu, nom. cons. - Back to Ranunculales
Plant sympodial; tannin 0, benzylisoquinoline alkaloids +, little oxalate accumulation; cork cambium deep-seated, rarely developed; when woody with broad primary rays persisting and cambium developing in the primary vascular bundles; nodes multilacunar; axillary bud vascular tissue derived from several leaf gaps [?level]; petiole bundes forming a ring; (cuticle waxes as platelets); stomata also paracytic; lamina margins usu. gland-toothed; flowers medium to large, P in two series, P and A not opposite each other; A many, spiral; tapetal cells 3-4-nucleate/polyploid; pollen surface spinulose-punctate; receptacle well developed, stigma ± dry; ovules several/carpel, apotropous, micropyle endostomal, parietal cells +/0; fruit a follicle; exotestal cells often thickened, unlignified, or seed ± pachychalazal, coat thin; endosperm starchy, embryo minute to short; x = 7 (?8), chromosomes [C-/Coptis type], nuclear genome [1C] (0.113-)2.554(-57.901) pg; germination epigeal, cotyledonary tube common.
62 [list, to tribes]/2,525 - five subfamilies below. ± World-wide, but mostly temperate.
Age. Anderson et al. (2005) estimated a crown-group age of ca 87-73 Ma for Ranunculaceae, Bell et al. (2010) ages of (73-)59, 55(-41) Ma, Wikström et al. (2001) ages of (91-)85, 65(-59) Ma, Fior et al. (2013) an age of (92.1-)87.3(-82.4) Ma, while (114.8-)108.8(-101.6) Ma is the age in W. Wang et al. (2016a) and ca 61.4 Ma in Jacques et al. (2011).
The recent discovery of Leefructus from early Cretaceous deposits 125.8-122.6 Ma old in China and assigned to stem Ranunculaceae (G. Sun et al. 2011; see also W. Wang et al. 2014a) may, if confirmed, very much change our ideas of the evolution of Ranunculaceae (see W. Wang et al. 2016a), Ranunculales, and perhaps of eudicots as a whole, but there seems to be some question about "the authenticity of the specimen" (sic: Z. Zhou 2014: p. 553). Gobo et al. (2022) recently described Santaniella from the fruiting/vegetative material found in the Crato Formation in northeast Brazil, and they thought that it had "potential affinities with ranunculids, and presumably Ranunculaceae" (ibid. p. 1), its age and locality being particularly interesting for such a core eudicot fossil. However, Pessoa et al. (2023a) obtained additional material of the genus, including material in bud, from the same locality, and suggested that it was more likely to be a mesangiosperm-magnoliid. However, they emphasized that the leaves and flowers in particular of Santaniella were difficult to interpret, and where in the magnoliids it might go was unclear - and even "some kind of magnoliid" might be too precise...
[Glaucidioideae + Hydrastidoideae]
: vessel elements also with scalariform perforation plates; stem with cortical + medullary bundles; vascular bundles flat; petiole with medullary bundles; palisade mesophyll 0; leaves two-ranked, lamina simple, vernation plicate, margin deeply palmately lobed; flowers single, terminal; nectary 0; androecial vascular supply fasciculate; stigma bilobed; nucellar cap massive; chromosomes large.Age. Bell et al. (2010) estimated crown-group ages for this clade of (66-)51, 48(-34) Ma and Wikström et al. (2001) ages of (80-)74, 47(-41) Ma.
1. Glaucidioideae Loconte - Glaucidium palmatum —— Synonymy: Glaucidiaceae Tamura
Coumarin +, alkaloids, berberin 0; lamina vernation supervolute-curved [conduplicate-wrinkled]; flowers with cortical vascular system; P = C, 4; A development centrifugal; G 2, basally connate, opposite outer P [transverse], grooved, stigma ?irregular-laminate; ovules many/carpel, micropyle endostomal, outer integument 6-13 cells across, with vascular bundle, inner integument ca 5 cells across, nucellar cap 15-20 cells across, parietal tissue 0; megaspore mother cells/embryo sacs several; follicle with stigma on lower abaxial surface, dehiscence thus also abaxial; seeds flattened, winged, outer integument vascularized; polyembryony common, embryo long, cotyledons foliaceous; n = 10.
1/1. Japan. Map: from H.-L. Li (1952), blue.
[Hydrastidoideae [Coptidoideae [Thalictroideae + Ranunculoideae]]: ancestral petal flat, with short claw. [?pretty much the whole order].
Age. This clade was estimated to be (88.8-)80.2(-70.8) Ma by J. He et al. (2022).
2. Hydrastidoideae Martynov - Hydrastis canadense L. —— Synonymy: Hydrastidaceae Martinov
Roots bright yellow; hydroxyquinoline alkaloids [hydrastine, berberine, etc.] +; nodes swollen, multilacunar; petiole base sheathing, leaves simple, vernation plicate; P uniseriate, small, deciduous, (2) 3 (4), with a single trace; A ?development; pollen tectum striate-reticulate; G several, stigma with multicellular projections; ovules (1-)2(-4)/carpel, apotropous, micropyle bistomal/zig-zag, outer integument 4-8 cells across, inner integument 2-4 cells across, parietal tissue 4-5 cells across, nucellar cap 8-10 cells across; fruit somewhat berry-like; seeds shiny, testa and tegmen multiplicative, exotesta strongly palisade, all other layers ± collapsed; embryo rather short; chromosomes short [0.5-2.5 µm long], n = 13.
1/1. Central and eastern North America. Map: above, from H.-L. Li (1952), red.
[Coptidoideae [Thalictroideae + Ranunculoideae]] / core Ranunculaceae: vascular bundles with xylem surrounding phloem [= amphivasal]; paratracheal parenchyma ± absent; petiole bundles with associated lignification; leaves (opposite, two-ranked), palmately compound, lamina vernation variable; inflorescence often cymose, or flowers single; P 5-merous, outer P = C, inner P nectariferous, stiptate [very diverse in form]; A development centripetal, extrose or introrse; tapetum glandular/amoeboid; pollen apertures low-level variation, tectum spinulose/punctate-microperforate; G (1-)many, usually with complete postgenital fusion, (ascidiate), when 3, orientation variable; ovules 1-15/carpel; outer integument 4-6 cells across, inner integument ca 2 cells across; antipodal cells enlarged/persistent, bi-/multinucleate; AQCOα whole genome duplication.
60/2,523. ± World-wide, but especially northern and montane. Map: from Vester (1940), Hultén (1971), Frankenberg and Klaus (1980), Trop. Afr. Fl. Pl. Ecol. Distr. 1 (2003) and Wilson (2007).
Age. Bell et al. (2010) estimated crown-group ages for this clade of (59-)45, 42(-30) Ma and Wikström et al. (2001) ages of (71-)66, 51(-46) My; (96.6-)89.9(-83.3) Ma are the ages in W. Wang et al. (2016a), ca 77.6 Ma in Y. Liu et al. (2021), (80.7-)71.7(-62.6) Ma in J. He et al. (2022) and (77.8-)75.6(-72.7) Ma in Y. Wang et al. (2022).
3. Coptidoideae Tamura
Small shrubs or perennial herbs; berberastine [alkaloid] +; petiole bundles embedded in lignified ring, (some bundles double - Coptis); flowers single/inflorescence (branched) raceme; C (-8), nectaries 5-10, capitate-clavate; pollen (pantoporate); carpels stipitate; n = (8) 9.
3/22: Coptis (15). East Asia, east and west North America.
Age. Crown-group ages for this clade are (20-)17, 12(-9) Ma (Wikström et al. 2001), (26-)16.2(-8.5) Ma (W. Wang et al. 2016a), ca 15.5 Ma (Xiang et al. 2018), or ca 16.6 Ma (J. He et al. 2022) - all Cop. Xan..
[Thalictroideae + Ranunculoideae]: outer integument 2-10 cells across, inner integument 2-3 cells across; postament + (0); (fruit an achene), (embryo medium).
Age. The age of this clade is estimated to be (75.9-)66.7(-58.1) Ma (J. He et al. 2022).
4. Thalictroideae Rafinesque / Isopyroideae Tamura, Isopyreae Schrödinger —— Synonymy: Aquilegiaceae Lilja, Thalictraceae Rafinesque
(Plants annual), (tussock-forming); tyrosine derived cyanogenic compounds, benzylisoquinoline alkaloids [esp. Thal.], 18:3[d]5trans,9cis,12cis, also 18:1[d]5trans, 18:2[d]5trans,9cis fatty acids +; petiole bundles embedded in lignified ring, medullary bundles +; hairs capitate; leaves to 3x compound, leaflet vernation ± curved-involute, ([adaxial] stipules + - Thal.); (plant dioecious); flowers (single), parts ± whorled (spiral - Leptopyrum]; K 3-10, petal-like, nectaries ± cup-shaped or with long spurs [Aquilegia - A.]/(C 0 - esp. Thal.); (internal staminodes + - Urophysa, laterally connate - A.); pollen (pantoporate - Thal.); G 1-20, stigma capitate-lingulate, papillate; ovule (1 carpel), integument single, 7-8 cells across [Leptop.], (obturator +); fruit (berrylet), (achene); (exotesta outer periclinal wall thickened - some T.); n = 6-9, x = 7, chromosomes short [0.5-2.5 µm long], often ± rod-like/simply curved, [T-/Thalictrum-type], 1C = >241.1 Mb, 35S sites proximal to centromere; chloroplast rpl32 gene moved to nucleus.
9/?330: Thalictrum (196), Aquilegia (80), Isopyrum (30). N. temperate, esp Asia (genera), also some South America, Africa and New Guinea.
Age. The crown-group age for this subfamily is (41.2-)33.8(27.6) Ma (W. Wang et al. 2016a: note topology), (28.7-)26.6(–24.5) Ma (Soza et al. 2013: Aq. + Th.), (32.3-)26.2(-20.3) Ma (Fior et al. 2013), ca 21.2 Ma (J. He et al. 2022) - or (70.3-)63.8(-60.5) Ma (Y. Wang et al. 2012: Iso. + Aquil.).
5. Ranunculoideae Arnott - note, gene trees largely discordant along this part of the spine.
(20:3[d]5cis,11cis,14cis fatty acid), benzylisoquinoline alkaloids usu. 0, berberine 0; (palisade mesophyll with arm cells); stomata ³35 µm long; hairs clavate; lamina segments ± involute (supervolute and/or curved); (A development centrifugal); tapetal cells 2≤ nucleate; (pollen multicolpate/pantoporate); ovule often 1/carpel, median, parietal tissue 1-2 cells across, (postament +); (seed coat poorly developed), (exotesta short-palisade), endotesta ± developed; endosperm nuclear, embryo various; n = 6-10, x = 8, chromosomes long [(3-)4.1-10.5(-12) µm long], 2-armed, arms often curved, [R-/Ranunculus-type], 1C = <43,708 Mb, 35S sites terminal or subterminal.
46/2025. Worldwide, but few in lowland tropics. [Photo - Flower.]
Age. Crown-group Ranunculoideae are estimated to be (156.4-)123.4(-94.5) Ma (J. Cheng & Xie 2014), ca 62.9 Ma (J. He et al. 2022) or ca 84.9 Ma (Ling et al. 2023: note topology).
Age. An estimate for the age of this clade is ca 57.7 Ma (J. He et al. 2022).
5A. Adonideae Kunth
(Ranunculin + - Trollius: ?level); nodes also 3:3 [Adonis]; leaves simple; P (= K-like), nectaries 3-17, oblong-linear, concavity at base/C-like, nectar 0; (tapetal cells 2-nucleate); pollen (tectum striate - T.); G with glands [herbivore deterrent - T.]; ovule lateral, unitegmic, bitegmic at micropyle. (obturator +); embryo sac bisporic, eight-nucleate [Allium type], (antipodal cells not enlarged); fruit an achene; exotesta ligniied, tanniniferous; n (= 12).
2/57: Trollius (31), Adonis (26). N. Temperate.
Age. Crown-group Adonideae are ca 27 Ma (J. He et al. 2022).
5B. Asteropyreae W. T. Wang & C. Y. Chang - Asteropyrum J. R. Drummond & Hutchinson
Plant shortly rhizomatous; ?nodes; ?petiole; leaves simple, peltate; flowers single; pollen pantocolpate/-porate; nectaries 5-8, long-stipitate, spathulate; n = 8.
1/2. Bhutan, N. Myanmar, S.W. China.
Age. The age of Asteropyrum + the rest is ca 82.4 Ma (Ling et al. 2023: note topology).
[Caltheae [Nigelleae [Delphinieae + Cimicifugeae]]]: ?
Age. This clade is estimated to be ca 60 Ma (J. He et al. 2022) or ca 76.0 Ma (Ling et al. 2023).
5C. Caltheae Berchtold & J. C. Presl - Caltha L. —— Synonymy: Calthaceae Martynov
Fatty acid profile; stipule +, adaxial; leaves undivided (with basal appendages); P (4-)5-10(-13); nectaries 0; anther wall monocot type; G 2-many, with ?nectar-secreting glands; antipodal cells multinucleate.
1/12. N. and S. Temperate.
[Nigelleae [Delphinieae + Cimicifugeae]]: ?
Age. The age of this clade is ca 59 Ma (J. He et al. 2022).
5D. Nigelleae Schrödinger - Nigella L. —— Synonymy: Nigellaceae J. Agardh
Annuals/biennials; nodes 3:3; flowers terminal (also on branches); nectaries [complex], tubular-bilabiate, abaxial lip much the longer; Ubisch bodies +; G [2-10] [connation varies]; n = 6 (7).
1/22. Eurasia, esp. Europe + the Mediterranean, not China.
[Delphinieae + Cimicifugeae]: ?
Age. The age of this clade is around 58.4 Ma (J. He et al. 2022).
5E. Delphinieae Schrödinger —— Synonymy: Aconitaceae Berchtold & J. C. Presl, Delphiniaceae Brenner
(Plants annual), (stem tubers); norditerpenoid alkaloids [e.g. aconitine], benzylisoquinoline alkaloids +; nodes 3:3; inflorescence racemose; flowers vertically monosymmetric; P = C-like, adaxial P spurred; 2 adaxial nectaries, (connate), spurred, nectar in spur, (+ 2, ± petal-like, 4, 6 or (8) rudimentary); (outer A staminodial); (micropyle endo-/bistomal - Delphinium); G (1), 3-5; whole genome duplication, n (= 12).
2/803: Delphinium (503), Aconitum (300). North temperate; tropical African mountains.
Age. Crown-group Delphinieae are ca 23.1 Ma (J. He et al. 2022).
5F. Cimicifugeae Torrey & A. Gray —— Synonymy: Actaeaceae Berchtold & J. C. Presl, Cimicifugaceae Bromhead
Triterpenoids +, fatty acid profile; (leaves simple); flowers single, involucrate/inflorescence racemiform or spicate; C 4-9(-10), nectaries 1-13+, (petal-like, ?not nectariferous), concavity at base, (paired apical appendages); pollen (pantoporate - Actaea); (tapetum amoeboid); ovule (unitegmic, bitegmic near micropyle); fruit (berrylet - A.); testa (undifferentiated - Eranthis), (tegmen thick-walled, pitted/not); n (= 7).
3/42: Actaea (32). North Temperate (subtropical).
Age. Crown-group Cimicifugeae have been estimated to be ca 29.8 Ma (J. He et al. 2022), although the estimate in Ling et al. (2023) is far older, (67.9-)63.2(-59.0) Ma.
[Helleboreae [Callianthemeae [Anemoneae + Ranunculeae]]]: ?
Age. The age of this clade is around 60.0 Ma (J. He et al. 2022).
5G. Helleboreae de Candolle - Helleborus L. —— Synonymy: Helleboraceae Vest
Bufadienolides [cardiac glycosides], (podophyllotoxin), ranunculin +; nodes 3:3; pollen tectum reticulate, Ubisch bodies +; nectaries obliquely tubular/cup-shaped; anther (epidermis with thickenings); C ± persistent in fruit; ovule unitegmic; chalazal/raphal elaiosome +.
1/21. Europe to China.
[Callianthemeae [Anemoneae + Ranunculeae]]: ranunculin + [lactone of 4-hydroxy-2,4-pentadienoic acid, broken down into glucose + toxic protoanemonin].
Age. This clade is ca 56.8 Ma (J. He et al. 2022).
5H. Callianthemeae W. Wang & Z. D. Chen - Callianthemum C. Meyer
?Nodes; ?petiole; flower single; P = ± K-like, 5-10, nectary C-like, 5-16, with basal pit; fruit an achene.
1/14. Europe to Japan.
[Anemoneae + Ranunculeae]: (petiole bundles arcuate); (tapetal cells binucleate); (pollen pantocolpate/-porate); ovule apotropous, unitegmic, integument 4-12 cells across, parietal cells often 0; fruit an achene; (embryo with one cotyledon).
Age. The estimated age of this clade is ca 50.4 Ma (J. He et al. 2022).
5I. Anemoneae de Candolle —— Synonymy: Anemonaceae Vest, Clematidaceae Martynov
Plant (monopodial - Anemone-Hepatica), liana, petiole climber, (evergreen)/deciduous); (complex secondary anatomy - Clematis0, (cork cambium deep-seated); nodes (3:3 - C.); petiole bundles separate, in arc or ring, cortex (and pith) lignified - C./medullary bundles - A.); leaves (opposite. pinnate - C.), (simple); (flowers terminal, (with involucre)); P uniseriate, C 4-20, (valvate), (outer and inner members differ somewhat - Knowltonia), nectaries 0 (from minute outer staminodes - Pulsatilla)/(few, clavate-spathulate, intergrade with A); anther (wall monocot), (epidermis with thickenings); (tapetum amoeboid); achene with persistent plumose style +/0, (berrylet); (seed coat with vascular bundle - A.); n = (7, 12); chloroplast IR with ca 4.4 kb expansion; (germination hypogeal, cotyledonary buds +, first leaves opposite, cataphylls, later leaves entire (lobed) - some C.).
Clematis (325), Anemone (90), Pulsatilla (40). Predominantly N. Temperate, but widely scattered elsewhere, including Oceania.
Age. Crown-group Anemoneae are ca 29.3 Ma (J. He et al. 2022).
5J. Ranunculeae de Candolle
(Plant annual), (stoloniferous); (vascular cambium 0); (cauline endodermis +); (nodes 3:3); leaves often simple; P often K-like, 5, (with basal spurs - Myosurus), nectary often C-like, (1-)5(-20), basal nectariferous pockets +, (series of pockets across C - Laccopetalum), (small, transversely bilabiate - Hamadryas), (0); (parietal cells + - Ceratocephala), (nucellar cap 0), (obturator +); (embryo sac bisporic, eight-nucleate [Allium type] - C.); n (= 7).
Ranunculus (1,616). N. Temperate, Arctic, to the Antipodes, tropical mountains.
Age. Ranunculeae may be ca 30.9 Ma (J. He et al. 2022).
Evolution: Divergence & Distribution. Paleoactaea, from the Late Palaeocene some 58 Ma, has fruits very similar to those of Actaea down to the palisade tissue in the testa (Pigg & deVore 2005). Somewhat older Eocaltha has seeds rather like those of extant Caltha, e.g. both have a flotation chamber; this fossil is from the Mexican Campanian (Cretaceous) some ca 77 Ma (Rodríguez de la Rosa et al. 1998; see also Pigg & deVore 2005 for early records), but its identity needs confirmation (Friis et al. 2011). If these fossils are placed in the crown groups of their respective genera, they will affect how we understand the evolution of the family as a whole.
A number of ages for Thalictrum are suggested by Soza et al. (2013) and for Ranunculoideae by Cheng and Xie (2014); the latter tend to be rather old.
Some relationships within the family (discussed below) and ages of clades have seemed rather up in the air. W. Wang et al. (2016a: q.v. for dates, c.f. topology, see below) suggested that the early evolution of the family took place in angiosperm-dominated forests during the Cretaceous Terrestrial Revolution (KTR) ca 109-90 Ma, and that all tribes had diverged by the end of the Cretaceous. They thought that around 83 Ma Ranunculaceae moved to non-forest habitats, eleven or so clades diverging within 1-14 Ma (Wang et al. 2016a). (Note that in Berberidaceae, sister to Ranunculaceae, the woody Berberis may be sister to the rest of the family (Wang et al. 2016a), and in Ranunculales as a whole there are other clades of herbaceous taxa.) On the other hand, Zhai et al. (2019) suggested that eleven tribes of their core Ranunculaceae - the tribes in Thalictroideae + Ranunculoideae above - are all early Palaeogene in age, diverging 65-50 Ma, while generic divergence occurred especially 30-15 Ma - in both cases perhaps because of isolation caused by fragmentation of cool habitats during warming periods. He et al. (2022) agreed that there seemed to have been these two waves of diversification, the first, the tribal wave, being characterised by "extremely complicated evolutionary processes, such as gene duplication, hybridization, introgression, and incomplete lineage sorting" (ibid. p. 14). Ling et al. (2023) used the evolution of Actaea as a sort of place-holder to get at the assembly of deciduous broad-leaved forests in general - they may have developed in the middle Eocene ca 43 Ma, the crown-group age that Ling et al. estimated for Actaea itself, with subsequent periods of further diversification, all during times of climatic deterioration.
The beginning of diversification within the speciose Clematis clade has been dated to (13.1-)7.8(-4.0) Ma, however, the stem age was estimated to be (43.8-)26(-9.2) Ma (Mikeda et al. 2006; Xie et al. 2011). The stem of the Coptis-Xanthorhiza clade may be as much as ca 55 Ma (c.f. Xiang et al. 2018), although it is thought that Coptis itself began diversifying ca 8.5 Ma (for details, see Y. Wang et al. 2022).
For the evolution of Arctic Ranunculaceae, see Hoffmann et al. (2010) and for that of sub-Antarctic Ranunculus in particular, see Lehnebach et al. (2017). Crown-group Ranunculeae may date to around (47.1-)39.5, 38.4(-28.6) Ma (W. Wang et al. 2014b, q.v. for other dates). In the widely-distributed Ranunculus there has been a substantial amount of dispersal to tropical and subtropical mountains and in the Southern Hemisphere - even between southern Africa and America - often followed by radiations (Emadzade et al. 2010, 2011; Hörandl & Emadze 2011), however, some of these dispersal events may be doubtful (Wang et al. 2014b: questionable sequences in earlier work). Other taxa that have moved to African mountains from the north include Dianthus and Carex (Gehrke & Linder 2009; Fassou et al. 2022).
Diversification within Aquilegia (Thalictroideae) has been much studied, the nectar spurs that characterise most of the genus being considered a key innovation that triggered recent and rapid diversification/adaptive radiation in the genus (Hodges & Arnold 1995 and references; Schenk 2021). There are only ca 80 species in the clade and they show little molecular differentiation (Whittall et al. 2006), and in a study of the 25 North American species, Whittall and Hodges (2007) found that there had been punctuational (at speciation) and directional (short→long spurs) evolution, the plants evolving to fit the morphologies of their pollinators - so not cospeciation s. str.. Bastida et al. (2010) suggested that sympatric speciation and pollinator shifts marked the evolution of Aquilegia in North America, while in Europe geographic isolation and shifts in habitat use were more prominent. Fior et al. (2013) thought that Aquilegia originated in Eastern Asia, whence it moved to Europe and (in the reconstructions they used) back to Asia, and also to America. See these authors for further details; diversification rates have been notably high in Europe within the last 1.7 Ma or so (see also Martín-Hernanz et al. 2019: Table 4). Pabón-Mora et al. (2013) suggested that aspects of floral development in Aquilegia differed from those in other members of the family; there the basal/abaxial member of alternating staminal rows is a long-spurred nectary which has three vascular traces running into it (for the spur, see also Wessinger & Hileman 2020). Ballerini et al. (2020) look at the control (by the POPOVICH gene) of cell proliferation during early spur development; amount of cell division, where it happens, and cell elongation all help shape the spur (M. B. Edwards et al. 2022). There are staminodes immediately surrounding the carpels, and Kramer et al. (2007) suggest that the evolution of these internal staminodes is connected with the duplication of B genes, while Sharma and Kramer (2012) provide evidence that two APETALA3 paralogues are involved. The members of the two staminodial whorls are laterally flattened, abaxially lignified, and adhere to one another - perhaps they have some protective role (Meaders et al. 2020, q.v. for developmental differences between the staminodes and stamen filaments).
Delphinium s.l., Aconitum, and relatives (Ranunculoideae-Delphinieae) have monosymmetric flowers and between them account for about a quarter of the diversity in the whole family. The flowers of Delphinium have a petal-like perianth, spirally arranged, and the adaxial member (or adaxial three members) of the outer perianth (sometimes called a whorl) has a spur inside which are one or two stalked nectaries of the inner "whorl"; for the spur, see e.g. Wessinger and Hileman (2020). There are frequently two asymmetrical petal-like structures adjacent to these nectaries; they are mirror images of one another and in D. anthriscifolium individually become resupinate - they are clawed, and the claws twist 180o, but in opposite directions, so preserving the mirror-image relationships of the two (W.-G. Zhang et al. 2021). This group is largely Mediterranean-East Asian in distribution, but with forays into Africa and North America. Delphinieae began diversifying early in the Oligocene (41.8-)32.3(-23.0) Ma, and the transition from a short-lived (± annual) to a perennial habit in Delphinium is associated with bursts of diversification (Jabbour & Renner 2011a, 2012a). Much diversification in the group has been in alpine habitats in the Himalaya-Hengduan region (Hughes & Atchison 2015), but rate shifts in Delphinieae from the Hengduan region are dated to ca 37 and 27 Ma, which predates the uplift of the Hengduan Mountains (Xing & Ree 2017, see also below for their pollinators). There has been duplication of Cycloidea genes involved in this monosymmetry, and these genes are variously expressed, ad- or abaxially, in the flower, and also in the outer whorl of the perianth (Jabbour et al. 2014; c.f. Hileman 2014).
Expression of a duplicated A-class gene, APETALA 3, is intimately involved in the development of the nectariferous petal-like structures found in Delphinieae and most other Ranunculaceae (see also Pollination & Seed Dispersal below), although there has been discussion as to what they "really" are - modified petals, or staminodes (e.g. Ning et al. 2023)? Absence of the gene has been linked to the loss of the nectarial function, and these nectary-type structures then look much more like petal-like sepals (R. Zhang et al. 2013; Gonçalves et al. 2013; Sharma et al. 2014). In a comprehensive survey, Delpeuch et al. (2022: esp. Figs 3A, 4B) found that a flat petal with a short claw could be considered the basic condition both in the family as a whole and in nearly all the tribes, although little is known about Hydrastis and Glaucidium, basal to the rest of the family. For the details of the development of the remarkable petals in Nigella, see P. Wang et al. (2015 - especially Yao et al. 2019; R. Zhang et al. 2020). In the latter two papers, the developmental repatterning involved in the formation of the remarkable petals in that genus - they put the labellum of Orchidaceae to shame - is described, many of the details of nectary morphology being linked to the activity of some thirty genes, including regulatory genes (all told some 918 genes were specifically expressed in the nectaries, some perhaps coopted from leaf and meristem development). Note, however, that remarkable as the floral morphology of Nigella is, the basic morphology of the ranunculacean petal is maintained, the nectary being at the adaxial base of the petal, and it is accessed by the pollinating bee that is guided by the paired, glistening pseudonectaries higher up the petal. There is as yet no evidence that these petals triggered a particularly noteworthy bout of speciation/diversification, indeed, if Nigella is sister to Delphinieae (see Phylogeny), it is not at all a speciose clade - 22 vs ca 700 species - indeed, the combined [Nigelleae + Delphinieae] clade all have rather complex nectaries... Q.-Q. Zhu et al. (2022) examined the diversity of petals with more or less elaborate nectar-secreting spurs in Isopyreae (= Thalictroideae) - of course, Thalictrum lacks petals entirely. Whittall and Hodges (2007) and Kramer and Hodges (2010) review the evolution of the rather petal-like spurs of Aquilegia (see Divergence & Distribution above). Variation in spur length, which is considerable, is not linked to changes in cell number (c.f. Linaria), which was roughly the same in the three species examined, rather, to the duration and direction of cell elongation (Puzey et al. 2012). The central part of the spur itself, at the bottom of which is the nectary, seems to be the result of the expression of auxin-related factors, normally expressed at organ margins, moving to a novel position (Yant et al. 2015). In the family as a whole the extensive variation in the numbers of floral parts and their morphology seems to be connected with the spiral phyllotaxis here, in whorled phyllotaxis the flexibility of the floral organ identity determination programme is reduced and floral development becomes canalized (P. Wang et al. 2015).
Zhai et al. (2019) and J. He et al. (2022) discuss the evolution of the distinctive chromosome morphologies in the family; see also below. Ranunculaceae have ca 170-fold genome size (Mbp) differences and in this are surpassed only by Melanthiaceae; in all other families the variation is well below 150-fold (Elliott et al. 2022b: Fig. S10). Apomixis is well known in Ranunculus in particular where it occurs in three to five groups, each with one or more extant diploid sexual species and one unknown progenitor species - all told 840 or more polyploid (mostly tetraploid or hexaploid) apomictic microspecies have been described in the largely European R. auricomus complex alone (Majeský et al. 2017; Karbstein et al. 2020, 2022).
Tobe and Keating (1985 and references, see Table 1) found a number of differences between [Glaucidium + Hydrastis] and other Ranunculaceae. However, they were not impressed by them, noting that 8/9 of them were also to be found in Podophyllum and Diphylleia, both Berberidaceae-Podophylleae.
Ecology & Physiology. Rhizomes and tubers of some Ranunculaceae perennate in a state of extreme dessication (Gaff & Oliver 2013). In some taxa shoots do not appear above ground every year, the phenomenon of vegetative dormancy (Shefferson et al. 2018; Hurskainen et al. 2018).
Many species of Clematis (inc. Naravelia, etc.) are lianes, sometimes very robust, that climb using leaflet or petiole tendrils, and, as with other climbers, the stems tend to become more flexible with age, the structural Young's modulus decreasing (Isnard et al. 2003a, b; see also S.-Z. Yang et al. 2021).
Pollination Biology & Seed Dispersal. Pellmyr (1995) summarized pollination in the family, and details of the development of some of the remarkable nectariferous structures in the family are to be found in Diversity & Distribution above - and I used to think of Ranunculaceae as having rather unspecialized flowers... In an analysis of European members of the family Waser et al. (1996) found as many as 53 species of pollinating insects from 29 genera visiting a single species - or as few as one. Caltha has nectariferous hairs on the carpels (e.g. Kapil & Jalan 1962), while taxa like Clematis and Ranunculus, the former lacking and the latter with nectar, have apparently unspecialized flowers and may be visited by many species of pollinators. However, many Ranunculaceae have distinctive nectaries that can take very different forms. Thus Delphinieae have monosymmetric flowers with single or paired nectary spurs borne inside a spurred petaloid member of the perianth whorl; Renner and Jabbour (2012b) and Zalko et al. (2020) discuss the evolution and development of this and other unusual pollination morphologies in the tribe. Bumble bees are the predominant pollinators of the 600-700 species of Delphinieae, a tribe that is very speciose in the Himalayas (Renner & Jabbour 2012b). Diversification of bumble bees, generalist bees that commonly visit morphologically specialized flowers and handle them quite easily (see below), probably occurred 40-25 Ma (Hines 2008 and references), i.e., about the same time as the diversification of Delphinieae themselves. Rather unusually for a bumble bee, Bombus consobrinus has specialized on Aconitum, especially on A. septentrionale, although several other bumble bees also pollinate members of that genus (Laverty & Plowright 1988; Thostesen & Olesen 1996); Kronfeld (1890: p. 19) early declared that Aconitum was an excellent example of an insect-adapted flower. Nigella has distinctive conspicuous glistening pseudonectaries on the inner perianth whorl of its flowers, and they help guide the pollinator to the nectaries proper which are found on the bases of these perianth members (H. Liao et al. 2020). For more on pollination, see Dötterl and Vereecken (2010).
The five, coloured nectar spurs of the polysymmetric flowers of Aquilegia are unusual in flowering plants, although similar flowers are found in Halenia, etc.. Nectar spurs are usually single, as in Delphinium (although there may be two spurred petals inside the outer calyx spur), rarely two separate spurs (Diascia), and are associated with monosymmetry. Melittophily is the basic condition for Aquilegia, but in North America ornithophily has evolved twice, with subsequent evolution of sphingophily (Thomson & Wilson 2008). Pollen deposition on the pollinator may be quite precise here (Kay et al. 2006b).
Filaments or perianth members of Thalictrum that are pollinated by insects (insect pollination is ancestral) have conical cells, micromorphological markers for petaloidy (Di Stilio et al. 2009). Many species are wind-pollinated (there is a correlation with polyploidy - Sosa et al. 2013), and some of these species are monoecious or dioecious, derived conditions (Kaplan & Mulcahy 1971; Soza et al. 2012; Goldberg et al. 2017), dioecy sometimes being cryptic (Timerman & Barrett 2018 and references). There are 8-10 origins of wind pollination in Thalictrum and perhaps two reversals to animal pollination (Soza et al. 2012; Wang et al. 2018). Taxa like T. pubescens are ambophilous, being pollinated both by wind and insects, and this condition may be quite common in the genus (Timerman & Barrett 2019; see also Martínez-Gómez et al. 2023: Table 1). Monoecy and dioecy are restricted to and predominate in species from the New World. The flowers, including those of at least some wind-pollinated taxa, have scent, but all lack nectar (T. N. Wang et al. 2018). Properties of the filament affect pollen release in wind-pollinated taxa; specifically, the amount of pollen released was negatively correlated with the natural frequency of oscillation of the filament, this stamen resonance strongly affecting stamen acceleration and hence pollen release (Timerman & Barrett 2018, 2019). (For the extensive effects of SEPALLATA E-class genes on floral morphology here, see Soza et al. 2016).
For the intimate association between Old World Trollius and its pollinators/seed parasites, the fly Chiastocheta (close to Botanophila), see Pellmyr (1992) and Ibanez et al. (2013: plant volatiles). Asparagaceae-Agavoideae, Phyllanthaceae, Saxifragaceae, Moraceae and Caryophyllaceae have similar interactions - see Hembry and Althoff (2016) and Kawakita and Kato (2017f) for reviews of diversification and coevolution.
A number of forest herbs in Ranunculoideae in particular are myrmecochorous, the outgrowths that attract ants developing either from the seed or the fruit (Lengyel et al. 2009, 2010).
Plant-Animal Interactions. North temperate Ranunculaceae are hosts to over 110 species of dipteran agromyzid leaf miners (Phytomyza: Spencer 1990; see also Jensen 1995), which for the number of species of Ranunculaceae involved may be the most diverse assemblage in flowering plants. Phytomyza (well over 700 species) may have moved on to Ranunculaceae from asterids, perhaps in the late Oligocene ca 24.5 Ma, and diversified there as the climate cooled; they have since moved back to asterids, especially to campanulid groups (Winkler et al. 2009).
Aquilegia eximia, which has sticky hairs, is protected from herbivores, including florivores, by carnivorous insects that are at least in part attracted to the plant by dead insects trapped by these hairs - this is by no mean unique, but this particular situation is the subject of a recent study by LoPresti et al. (2015), and can lead to the plant getting nitrogen indirectly from the trapped insects.
Plant-Bacterial/Fungal Associations. Mucoromycote fine root endophytes seem to be particularly common on Ranunculaceae growing in the Canadian Arctic (Orchard et al. 2017).
Genes & Genomes. A genome duplication in Aquilegia has been dated to (60.4-)51.1(-44.8) Ma (Vanneste et al. 2014a), so it may be fairly deep in the family. Y. Liu et al. (2021) thought that the AQCOα whole genome duplication was also to be found in Coptis; immediate outgroups beyond that have not been examined. However, J. He et al. (2022) placed a duplication event at the [Coptoideae + the rest] clade and at Delphineae; duplications possibly characterised Ranunculoideae and Anemoneae. He et al. (2022) also suggested that there had been an ancient hybridization event between Trollieae (R-type chromosomes) and Thalictroideae (T-type chromosomes), and also noted other possible hybridization events.
It is over 80 years since Langlet (1932, see also 1927) realized that the cytological variation in the family had a stong systematic signal, genera either having large R(anunculus)- or small T(halictrum)-type chromosomes, a finding that was at odds with the then-accepted classification. Important subsequent studies include those of Lewitsky (1931) and Gregory (1941). Gregory (1941) added a third chromosome type, the C(optis) type, which had "distinctly long and slender rather than bean-shaped" (ibid.: p. 490) chromosomes. Although Tamura (1995) and Yuan and Yang (2006) thought that the C type was best included in the T type, Zhai et al. (2019) recognized the former and talked about its evolution... Okada and Tamura (1979) noted characters other than gross size and shape that also separate the two chromosome types (see also Tamura 1993). Yuan and Yang (2006) added karyotypes for a number of taxa and also commented on some earlier findings. However, the correlation between chromosome morphology and taxonomy sometimes breaks down; Chung et al. (2013: note lengths given for the two types) found that the chromosomes of some species of Ranunculus like the annual R. sceleratus were quite small, rather like those of T-type chromosomes, and Eranthis also has quite small chromosomes (Gregory 1941). J. He et al. (2022:Glaucidium not included) also recognized three chromosome types; they thought these types had nothing to do with gene duplications in the family.
Baltisberger and Hörandl (2015) looked at karyotype evolution in Ranunculus and its immediate relatives, while Filiaut et al. (2018) examined the Aquilegia genome, finding a rather high (and inexplicable) level of polymorphism on chromosome 4, as also in Semiaquilegia. Sosa et al. (2013) discuss recurrent polyploidy in Thalictrum (to 24x); genome size seems to be independent of chromosome number, some diploids increasing in size and polyploids decreasing, indeed, genome size is quite small here (1C = 0.25–3.7 pg); for genome size in Isopyreae (= Thalictroideae), see also J. He et al. (2022).
Apomixis is well known in the Ranunculus auricomus complex, which is probably formed from five diploid sexual species (one is likely to be extinct) and includes some 840+ apomictic microspecies (Hörandl et al. 2007; Hodac et al. 2018, 2019; Karbstein et al. 2022 and references). Apomixis and the associated lack of recombination can lead to the accumulation of deleterious mutations, Muller's ratchet, although a mere 5% of sexual reproduction counteracts such deleterious genetic effects - and indeed apomixis is often not obligate (e.g. Hodac et al. 2019).
H. Liu et al. (2018) found a fair amount of variation in the chloroplast genome in Anemoneae, with a notable expansion of the inverted repeat that is associated with duplication of six ribosomal protein (rps) genes. Although the rpl32 gene is also found in the chloroplast in Thalictroideae, it is non-functional there (Park et al. 2015b).
Mlinarec et al. (2016a) examined retrotransposon (Tekay chromoviral elements) and genome evolution in Anemone s.l. and found a correlation between the two.
Chemistry, Morphology, etc.. Benzylisoquinoline alkaloids are largely absent from Ranunculaceae, although present in Coptis and Isopyyrum (Coptidoideae: Jensen 1995), which makes placing this feature on the tree difficult (lost and regained versus two losses). Ruijgrok (1966) clarified the distribution of the lactone ranunculin and of cyanogenic compounds.
The vascular bundles often have xylem surrounding the phloem, but c.f. Takhtajan (1997). Clematis, secondarily woody, has storied wood (see Carlquist 1995a for wood and bark anatomy); it is a liane with opposite leaves with sensitive, twining petioles. There are cortical bundles in the erect stem of Hydrastis, but not in the rhizome; the rhizome ofGlaucidium is an irregular sympodium. The development of the leaflets in the compound leaves of Aquilegia is acropetal (Hagemann & Gleissberg 1996). Variation in petiole anatomy is extensive (Tamura 1962, 1995) and adaxial/intrapetiolar stipules occur sporadically in the family (Hagemann 1970).
Monosymmetry in flowers of Delphinieae becomes apparent only rather late in development after organ initiation has begun (Jabbour et al. 2009a). Soltis et al. (2003a) suggested that bothGlaucidium and Hydrastis have a bimerous perianth. Floral phyllotaxy in Anemoneae is particularly variable, transitions between various spiral and whorled arrangements occuring with variation in numbers of perianth parts, even within a species (Ren et al. 2010; Kitazawa & Fujimoto 2018).
For an early discussion on stamens and nectaries in Ranunculaceae, and a suggestion that the flower might be fundamentally 3-merous, see Salisbury (1919). Nectaries in the family vary greatly in morphology from small and almost goblet-like to ± complex petal-like structures, and they are generally thought to be modified stamens. The two have a number of points of similarity, e.g. the "petals" have a single trace, although in some Delphinieae they have two traces (Novikoff & Jabbour 2014: two members connate?). They are in the same parastiches as androecial members, are similar to stamens in early development, are often peltate, originate from a primordium that is a mound rather than a ridge, and there are sometimes intermediates (Jäger 1961; Tamura 1965; Kosuge & Tamura 1989; Erbar et al. 1999; Leins 2000; L. Zhao et al. 2011; c.f. Kosuge 1994). Normally the nectaries are rather different morphologically from the single perianth whorl and are sometimes quite elaborate beaker- or hood-shaped structures; the single perianth whorl is usually petal-like and visually attractive. However, in Ranunculus and Ficaria the perianth whorl is green and protective, while the nectaries are very petal-like, the nectary proper being a small scale at the base of what otherwise appears to be an ordinary petal, while in Laccopetalum and relatives there are a number of nectary ridges across the width of the petal-like nectary; in the latter group both petals and stamens may have three traces (Hiepko 1964a). For the long-spurred nectary of Aquilegia , see above. Genera like Clematis, Thalictrum and Anemone s.l. may lack nectaries/petal-like structures adaxial to the petal-like sepals, but in some species of Anemone the carpels are nectariferous and in some Clematis nectar is secreted by the innermost stamens (Erbar et al. 2014). For spurs and different modes of nectar secretion within Ranunculoideae, see also Anton and Kaminska (2015).
In a number of taxa there is an 3-leaved involucre below the single flower, and in Anemone s.l. this is sepal-like, especially evident in the Hepatica group where this involucre is borne immediately underneath the flower with its petal-like perianth, although it does not particularly closely envelop the rest of the flower. These "sepals" have only a single trace and there are no nectaries. W. Wang and Chen (2007) discuss "petal" evolution in Thalictroideae; see also above, again, petals/nectaries have been lost. In Aquilegia the stamens are in ten vertically-arranged two-ranked series, each opposite an internal staminode, unique to Ranunculales (see Divergence & Distribution above), and their maturation is basipetal (see Remizowa 2019: other Ranunculaceae?). Insertion of the stamens, etc., can be spiral or whorled (Gonçalves et al. 2013). Tamura (1996) described the androecial development ofGlaucidium as being centrifugal and the androecium as being innervated by branches of staminal trunk bundles, very like the androecial development common in polystaminate core eudicots. See Erbar (2010) for more on androecial and nectary development.
Laccopetalum has huge flowers up to 15 cm across and with ca 10,000 carpels. Although the carpels of Nigella are more or less connate, no compitum is developed (Erbar 1998), indeed, no compitum of any sort has been recorded from the family (e.g. X.-F. Wang et al. 2011). There are often five traces to each carpel. When there is only one ovule/carpel, it is the basal member of the series (c.f. Rosaceae, with which Ranunculaceae share a superficial similarity, but where the single ovule is the apical member of the series). Uniovulate taxa are usually also unitegmic and have a nucellar cap (Philipson 1974). Bouman and Calis (1977) give details of the integuments of some Ranunculoideae. Z.-F. Wang and Ren (2008) suggested that unitegmic ovules have arisen in different ways, the single integument being either the outer (e.g. Clematis) or the inner integument (e.g. Ranunculus); they also described a rather obscure annular structure that surrounds the ovule in Coptis. The adaxial side of the carpels ofGlaucidium grows more than the abaxial as the fruit develops, so the stigma ends up on the "lower" surface; there the embryo is shown as being long by Tamura (1972) and Takhtajan (1988), but it is described as being minute by Takhtajan (1997). There is indeed extensive variation in embryo size (Tamura & Mizumoto 1972) and seedling morphology; the development of a cotyledonary tube is quite common in the family, while Ranunculus ficaria, for example, has only a single cotyledon (Förster 1997).
For general information, see Marié (1885), Kumazawa (1937b), Johri et al. (1992), Tamura (1993: Glaucidium excluded, he thought that it could not be a member of the family - see p. 570), 1995a, b: includes infrageneric groupings), Tobe (2002: Hydrastidaceae, includes Glaucidium) and Grey-Wilson (2023: Paraquilegia), also Hegnauer (1969, 1986, 1990, 1995) and Jensen (1995), all chemistry, Hao et al (2018: chemistry and medecine), Aizetmüller (1995, 1996) and Aizetmüller et al. (1997b, 1999) (fatty acids), Dulin and Kirchoff (2010: wood anatomy), Ezelarab and Dormer (1963) and Kavathekár & Pillai (1976), both nodal anatomy, Tamura (1962: petiole anatomy), Kumazawa (1937: leaf vernation), Jabbour et al. (2015: floral terata), Schöfel (1932: esp. floral diagrams), Brouland (1935: floral vasculature), Mair (1977: development, monosymmetry), Luo (2020: Leptopyrum), Rohweder (1967a: carpels), Huss (1906), Bhandari (1967 and references), and Engell (1995), all embryology, van Heel (1981, 1983: carpel development), Trifonova (1990 and references: petiole and seed anatomy), Weberling (1989: nectaries), G. H. Smith (1928), Endress (1995a), Tucker and Hodges (2005: Aquilegia and relatives), Leins and Erbar (2010), Ren et al. (2009: Ranunculoideae-Adonideae, 2011: Thalictroideae), and L. Zhao et al. (2011, 2012, 2016a: some Ranunculoideae), all floral (and some inflorescence) morphology, Nowicke and Skvarla (1983b: Helleborus), Xie and Li (2012: Clematis) and Humphrey and Ossip-Drahos (2018), all pollen, X.-q. Wang et al. (1993, also paper before it) pollen and seed, Eckardt (1957), gynoecium, Bhandari and Asnani (1968) and Y. Yang et al. (2019), embryology, for fruit and seed morphology and anatomy, see Aydin and Dönmez (2019: Nigella seed morphology), Ghimire et al. (2015a: Ranunculoideae) and Jung and Heo (2017) and Ahn et al. (2023), both Korean taxa, and for the distinctive seedlings of some species ofClematis, see Essig (1991). See Tobe and Keating (1985) and Tobe (2002) for Hydrastis, Tamura (1972) provide much information on Glaucidium, embryology mostly from Kumazawa (1938a), but see also Tobe (1981, 1995: also seed, etc.).
Phylogeny. For early work on Ranunculaceae, see Hoot (1991, 1995) and Jensen et al. (1995). The clade [Hydrastis +Glaucidium] was found to be sister to the rest of the family by Hoot et al. (1998) and some others since. This and other major phylogenetic structure within the family - [Coptidoideae [Thalictroideae + Ranunculoideae]] - might seem quite well established (c.f. also in part Ro et al. 1997; W. Wang et al. 2005). However, things seem rather up in the air, the recent study by Zhai et al. (2019) indeed resulting a tree that has quite good support for the most part, but it is based on plastome data alone and the sampling (all tribes, but only 31 of the ca 62 genera included) is marginal by current standards. Thus W. Wang et al. (2009) found strong molecular support for the relationships [Glaucidium [Hydrastis + rest of Ranunculaceae]], that for [Hydrastis + rest of Ranunculaceae] being weakened slightly by the addition of morphological data, and there was weak support for this topology in Hoot et al. (2015), (strong) support in W. Wang (2016a), Zhai et al. (2019), Carrive et al. (2020), and this topology was also recovered in the Angiosperms353 data set (see the Seed Plant Tree of Life version i.2022); Soltis et al. (2011) found some support for a topology [Hydrastis [Glaucidium + Ranunculus]] (the only three taxa of Ranunculaceae in their analysis). Wang et al. (2016a: 6 genes, good sampling) found a rather different set of relationships in the core of the family, [Glaucidium [Hydrastis [Coptidoideae [Adonideae [Nigella [Thalictroideae + other Ranunculoideae]]]]. See also Z.-D. Chen et al. (2016) for relationships among Chinese taxa of the family; [Coptis [[Eranthis + Cimicifuga] [Asteropyrum [[Thalictrum + Caltha] [other Ranunculoideae]]]]] were the relationships obtained. Similarly, Cossard et al. (2016), using eight markers from all three genomic compartments, found the relationships [Glaucidium [Hydrastis [Coptidoideae [[Adonideae + Thalictroideae] [other Ranunculoideae (includes Nigella)]]]], although support for the position of Adonideae was weak and that for [other Ranunculoideae] not too strong, either. Lehtonen et al. (2016) also recovered the relationships [Glaucidioideae [Hydrastidoideae ...]]. Similarly, H. Liu et al. (2018) and especially Zhai et al. (2019), both analysing chloroplast genomes, found moderate/strong support for an [Adonideae + Thalictreae] clade. Hence the relationships above should be interpreted with caution, for example, the vegetative and anatomical similarities between Glaucidium and Hydrastis are quite extensive, and if the two do not form a clade, as seems likely, these might be characters, perhaps apomorphies (using simple parsimony, ACCTRAN), for the whole family.
J. He et al. (2022: 54 samples, all tribes except Glaucideae, phylotranscriptomic/RMA-seq studies) carried out a variety of analyses on the family. The monophyly of all tribes in which more than one species was included was well supported, and although other relationships in the species tree were mostly well supported, gene trees were largely discordant along the [[Adonideae + Asteropyreae] [Caltheae [Nigelleae [Delphinieae + Cimicifugeae]]]] part of the spine. Comparing relationships in nuclear and plastome trees, there were some differences, most notably, Adonideae (R-type chromosomes) were sister to Isopyreae (Thalictroideae) (T-type) in the plastome tree despite their different chromosome morphologies, but this perhaps reflects an ancient hybridization/introgression event; Cimicifugeae were also migratory, and the relationships of Helleboreae + Delphinieae, Nigelleae, and within Anemoneae also varied (He et al. 2022).
For relationships within Thalictroideae, see Ro and McPheron (1997), W. Wang and Chen (2007) and Park et al. (2015b). The latter found the clade [[Isopyrum + Enemion] [Aquilegia + Semiaquilegia]] to be sister to other Thalictroideae, although Zhai et al. (2019) found that clade to be sister to Dichocarpum in particular, although with very little maximum parsimony support. Fior et al. (2013: 62 spp., extensive plastid data) looked at the phylogeny of Aquilegia (see also under Diversity & Distribution above). Relationships along the spine of Thalictrum are for the most part poorly supported, but support is good for an insect-pollinated clade including T. thalictroides and T. rubescens being sister to the rest; current sections (14) seem largely useless (Soza et al. 2012, 2013; see also K.-L. Xiang et al. 2022: some plastomes; Martínez-Gómez et al. 2023: 93 spp., 6 chloroplast markers).
Ranunculoideae. Relationships found by Zhai et al. (2019) are [[Adonideae, Asteropyreae, Caltheae [Nigelleae + Delphinieae]] [Cimicifugeae [Callianthemeae [Helleboreae [Ranunculeae + Anemoneae]]]]], the support being mainly from ML bootstrap and Bayesian analyses, (much) less from the maximum parsimony bootstrap analysis.
Adonideae. W. Wang et al. (2010) discuss relationships here.
Anemoneae: Hoot and Palmer (1994), Hoot et al. (1994, 2004, 2012; 55 taxa, 1 nuclear and 1 chloroplast markers), Schuettpelz et al. (2002), Meyer et al. (2010) and H. Liu et al. (2018) discuss relationships in Anemone s.l., which includes Hepatica, Pulsatilla, etc.; there is a considerable amount of pollen variation in the clade (e.g. Ehrendorfer et al. 2009) and relationships do not yet seem to be settled (Liu et al. 2018), although it is likely that Anemone s.l. is paraphyletic (see also Carrive et al. 2020). Sramkó et al. (2019: nucleus + plastid) looked at relationships within Pulsatilla and found the morphologically quite distinctive P. kostyczewii to be sister to the rest of the genus, with moderate support. In Clematis, Xie et al. (2011; see also Mikeda et al. 2006) provide a fairly comprehensive analysis, unfortunately, several of the deeper branches in the genus are poorly supported, and the main clades that are evident neither correlate very well with previous infrageneric taxa nor have much morphological support. However, Lehtonen et al. (2016: 132 taxa, 4 chloroplast and nuclear ITS markers, 40 morphological characters) found 12 stable clades within Clematis, some earlier sections seemed to be holding up, even if their relationships were not (c.f. Johnson 2001). Xiao et al. (2022: 32 spp.) compared plastome and nuclear analyses in Clematis, finding substantial differences in the topologies of the two.
Cimicifugeae. For the phylogeny of Actaea, see Compton et al. (1998a, b), its limits are to be extended; Ling et al. (2023: four plastid plus nuclear ITS markers) looked at all the species in the genus.
Delphinieae. There was little resolution of relationships within the speciose Delphinium section Diedropetala (Koontz et al. 2004). Luo et al. (2005) discuss the phylogeny of Aconitum subgenus Aconitum. Jabbour and Renner have greatly clarified relationships around here. The clades that Jabbour and Renner (2011a, 2012a; also W. Wang et al. 2013) found only partly mapped on to previously-recognized genera. Consolida and Aconitella were embedded in Delphinium, Aconitum gymnandrum, although belonging in this tribe is near-basal and did not link with any major clade, while D. staphisagria was sister to all other Delphineae. Xiang et al. (2017) discussed the limits of and relationships within subgenus Delphinium.
Ranunculeae. Relationships around Ranunculus are interesting. Ficaria, Myosurus, with its very elongated receptacle and as a result a flower that looks like the inflorescence of Houttuynia (Saururaceae), and [Laccopetalum + Krapfia], with their relatively large to huge flowers, many carpels, polyporate pollen, an androgynophore, etc. (Lehnebach et al. 2007) form a basal grade in a strongly supported clade with a monophyletic Ranunculus - see Hörandl et al. (2005), Paun et al. (2005), Hoot et al. (2008), Gehrke and Linder (2009: African montane taxa), Emadzade et al. (2011, but see W. Wang et al. 2014b in part) and Baltisberger and Hörandl (2015).
Classification. The back-bone of the classification above is largely based on that in Tamura (1993), Jensen et al. (1995) and especially J. He et al. (2022). Glaucidium has quite often been placed in its own family (indeed, it was excluded from Ranunculaceae by Tamura), but it would be monotypic; although a distinctive plant, it has quite a lot in common with Hydrastis (see also Cai et al. 2010, c.f. in part Cai et al. 2009). However, just looking at the variety of relationships of the major clades within the family that had been suggested over the last twenty years or so (e.g. Cossard et al. 2016: Fig. 3) there was clearly no consensus over many tribal/subfamilial groupings in the Ranunculoideae-Thalictroideae area in particular - the tribes themselves might be o.k., but not much else was. It is hoped that with He et al. (2022) relationships will settle down - note that they recognized only tribes in the family, while above subfamilies from some older classifications are included.
There are a number of problems with generic limits; see E. Welk in Kadereit et al. (2016) for a summary of some of these. For generic limits around Ranunculus, see Emadzade et al. (2010), in Adonideae, see W. Wang et al. (2010), and around Anemone, see Hoot et al. (1994), Ehrendorfer (1995) and Hoot (1995). This latter problem has still not been fixed. Thus Pfosser et al. (2011) suggested that Anemone may be best divided into two, one clade having x = 7 (inc. Hepatica) and the other x = 8 (see also Zhang et al. 2015; Mlinarec et al. 2016b: 5S rDNA). There is certainly a lot of variation around here in bract morphology, staminode presence/absence, pollen morphology, etc. (Ziman et al. 2008 and references), and although the genus is currently being dismembered (see Mosyakin 2016, 2018 and references), Lehtonen et al. (2016) and H. Liu et al. (2018) suggested that the idea of putting all Anemoninae into Anemone s.l. was questionable. Although Hoot et al. (2012) had supported the dismemberment of Anemone, there are outgroup problems there - they provided a detailed infrageneric classification of Anemone s.l.. For an infrageneric classication of Pulsatilla, see Sramkó et al. (2019). In the Delphinium area, Aconitella is derived from within Consolida, and the combined clade is to be included within Delphinium (Jabbour & Renner 2011a, 2012a; see also Jabbour et al. 2011; Xiang et al. 2017: infrageneric classification); Staphisagria is to be resurrected (Jabbour & Renner 2011b) and Pseudodelphinium is a peloric form of Delphinium (Espinosa et al. 2017). Actaea is to include Cimicifuga (Compton et al. 1998b).
Previous Relationships. Ranunculaceae are a classic example of a "famille par enchaînement", nothing in particular seeming to hold them together, but work over the last two decades suggests that they are largely monophyletic. However, Paeonia, quite often associated with Ranunculaceae in the past, is now included in Saxifragales as Paeoniaceae, while Tamura (1972) thought thatGlaucidium was close to Hypericales (= Malpighiales).
Botanical Trivia. The zygote of Anemone flaccida is undivided at the time of seed dispersal (Tamura & Mizumoto 1972).