EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
EXTANT GYMNOSPERMS / ACROGYMNOSPERMAE
Biflavonoids +; ferulic acid ester-linked to primary unlignified cell walls, silica usu. low; root apical meristem organization?, protophloem not producing sieve tubes, with secretory cells, sieve area of sieve tube with small pores generally less than 0.8 µm across that have cytoplasm and E.R., joining to form a median cavity in the region of the middle lamella, Strasburger/albuminous cells associated with sieve tubes [the two not derived from the same immediate mother cell], phloem fibres +; sclereids +, ± tracheidal transfusion tissue +, rays uniseriate [?here]; stomatal poles raised above pore, no outer stomatal ledges or vestibule, epidermis lignified; cuticle waxes as tubules, nonacosan-10-ol predominates, n-alkyl lipids scanty; buds perulate/with cataphylls; leaves simple, lamina 1-veined, development marginal; plants dioecious; parts of strobili spirally arranged; microsporangia abaxial, dehiscing by the action of the epidermis [= exothecium]; pollen saccate, tectate, endexine lamellate at maturity all around grain, esp. intine with callose; ovules aggregated into compound strobili, erect, pollen chamber formed by breakdown of nucellar cells, nucellus massive; ovules increasing considerably in size between pollination and fertilization, but aborting unless pollination occurs; ovule with pollination droplet, catches pollen; male gametophyte: two prothallial cells + tube cell + antheridial cell, the latter → sterile cell + 2 gametes; pollen germinates on ovule, usu. takes two or more days, tube with wall of pectose + cellulose microfibrils, branched, growing at up to 10(-20) µm/h-1, haustorial, breaks down sporophytic cells; male gametes released by breakdown of pollen grain wall, with >1000 cilia, basal body 800-900 nm long, spline hundreds of tubules wide, chromatin not condensed; fertilization 7 days to 12 months or more after pollination, to ca 2 mm from ovule surface to egg; seeds "large" [ca 8 mm3], but not much bigger than ovule, with morphological dormancy; testa mainly of coloured sarcoexotesta, scleromesotesta, and ± degenerating endotesta; first zygotic nuclear division with chromosomes of male and female gametes lining up on separate but parallel spindles, embryogenesis initially nuclear, embryo ± chlorophyllous; gametophyte persists in seed; plastid and mitochondrial transmission paternal; genome size [1C] 10< pg [1 pg = 109 base pairs]/(2201-)17947(-35208) Mb; two copies of LEAFY gene [LEAFY, NEEDLY] and three of the PHY gene, [PHYP [PHYN + PHYO]]; plastome IR expanded, with duplicated ribosomal RNA operons; chondrome with second intron in the rps3 gene [group II, rps3i2].
[CUPRESSALES [GNETALES + PINALES]] / PINOPHYTA / CONIFEROPHYTA / CONIFERAE / PINOPSIDA - Back to Main Tree
Tree, branched, evergreen; βaryl ether concentration in lignin lower, biphenyls higher; compression wood + [reaction wood - much-thickened/lignified fibres on abaxial side of branch-stem junction]; wood pycnoxylic, torus:margo pits + [tracheid side walls]/(0), Bars of Sanio/crassulae thickenings +; phloem with polyphenol-containing parenchyma (PP) cells, resin canals/cells in phloem and/or xylem + (0); lignins with guaiacyl units (G-lignin) [lacking syringaldehyde, Mäule reaction negative]; cork cambium ± deep seated; bordered pits on tracheids round, opposite; nodes 1:1; axillary buds + (0); leaves with single vein, fasciculate or not, needle-like or flattened; plants monoecious; microsporangiophore/filament simple, hyposporangiate; dehiscing by the action of the hypodermis [endothecium]; pollen saccate, exine thick [³2 µm thick], granular; ovulate strobilus compound, erect, ovuliferous scales flattened, ± united with bract scales [= ovules borne on predeveloped axillary structures]; ovules with pollination drop, lacking pollen chamber, inverted [micropyle facing cone axis at pollination and seed dispersal]; male gametophyte: pollen buoyant, not wettable, after pollination only male gametes produced [?here], pollen tube unbranched, growing towards ovule, growth intercellular, not haustorial, wall with cellulose and arabinogalactan proteins; gametes non-motile, lacking cell walls, siphonogamy [released directly to the egg cell], discharge distal; female gametophyte: lacking chlorophyll; seed cone components sclerified, seed dispersed with part of supporting structure, seed coat dry, not vascularized; early embryogeny: initially with 2 to 5(-6) free-nuclear divisions, elongated suspensor cells +, embryonal cells basal; polyembryony +; one duplication in the PHYP gene line; germination phanerocotylar, epigeal, (seedlings green in the dark).
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
N.B.: "Conifers" in the discussion below refers to Pinales and Cupressales together, unless otherwise mentioned; Gnetales are not usually included. For Cupressales, see below, while the Pinales, as well as Gnetales and the [Pinales- + Gnetales] clades are discussed elsewhere. Note that Page (2024) calls woody/arborescent gymnosperms, i.e. Ginkgoales, Pinales and Cupressales here, "resinifera"
Age. Clarke et al. (2011: also other ages) suggested a crown age for this clade of (286-)252(-212) Ma, Magallón et al. (2013) an age of ca 278 Ma, Won and Renner (2006) an age of (324-)298(-270) Ma, while ca 290 Ma is the estimate in Ran et al. (2018a). The estimates by Crisp and Cook (2011) of around 270 Ma and of around 260.9 Ma by Tank et al. (2015: Table S2) are broadly comparable. Leslie et al. (2012) suggested an age of around 350-275 Ma, while in Leslie et al. (2018) the suggestion was (329.3-)311.1(-287.9)) and in Ran et al. (2018b) it was (312.6-)276.2(-206.7) Ma, but Zhou et al. (2014) and Magallón et al. (2015) offered appreciably younger ages of (187.3-)161.4(-147) and ca 127 Ma respectively while the estimate in Herting et al. (2020) is (466-)346(-252) Ma; see also P. Soltis et al. (2002). Note that Gnetales were not included in many of the trees on which these estimates were based.
Evolution. Divergence & Distribution. What follows is rather incomplete, most notably, the rich fossil record of stem [Cupressales [Gnetales + Pinales]], of the three orders themselves, and most of that of the gymnosperms as a whole, has largely been ignored.
Leslie et al. (2012, see also 2018) offer ages for many conifer clades (see below) and evaluate the fossil data critically. The four- and three-gene trees (respectively) they produced are based on a very good sampling of the group; in the latter study 578 species were included, ca 90% of the total, although 18S sequences were obtained from only 126 taxa, less than a quarter (Leslie et al. 2018).
It has been suggested that the evolution of serotiny, release/dispersal of seed being a response to an environmental trigger, in this case fire, may have been of central importance in the evolution of conifers in general. He et al. (2015) dated stem-group conifers (= crown-group gymnosperms minus Gnetales) to (346-)332(-311) Ma, and the conifer relatives like Cordaitales and Voltziales evolving soon after then in the high oxygen atmosphere of the later Palaeozoic were all serotinous, having a compact cone with a woody axis, winged seeds and woody scales (although their sporophylls seem not to have been woody, at least some had secondary xylem - He et al. 2015: Fig. 3C). Similar cones are of course to be found in all extant conifer groups except Taxaceae and Podocarpaceae, and reversals from the serotiny syndrome are also to be found in fossil clades evolving later in the Mesozoic when oxygen concentrations were lower (He et al 2015, see also Berner 2009; Belcher 2010b).
Of the four major clades of conifers, Pinaceae (= Pinales) are mostly northern, while in Cupressales, [Araucariaceae + Podocarpaceae] are now mostly southern, and there are two major clades in Cupressaceae, Actinostroboideae (they used to be called Callitridoideae) and Cupressoideae, that have largely inhabited the southern and northern hemispheres respectively since the Jurassic (Brodribb et al. 2012). The current distributions of many extant conifer groups is much more restricted than and/or very different from their past distributions. Thus McIver (2001) reported fossils of the African Widdringtonia (Cupressaceae) from rocks of Cretaceous age in Alabama (for the distributions of all conifers, see Manchester 1999 for north temperate distributions; Farjon & Filer 2013). Many conifers have fossil records going back to the Cretaceous; for the early Caenozoic fossil history of what are now East Asian endemics, see Ferguson et al. (1997) and Manchester et al. (2009) - genera in Taxaceae, Pinaceae, Sciadopityaceae and Cupressaceae are included. However, Biffin et al. (2010b) note that some calibration scenarios have crown-group divergence of Araucariaceae and Podocarpaceae largely a (mid-Cretaceous to) Caenozoic phenomenon, which would question the attribution of early fossils to crown groups of/in those families.
Sundaman et al. (2019) looked at biodiversity hotspots in conifers in general, noting that 80% or more of the species in them are not restricted to these hotspots, which are in stable mountainous areas; they are areas where species accumulate, as much refugia as anything else (see also Harrison & Noss 2017 in part, in southwest Australian angiosperm groups like Proteaceae, etc.). These hotspots include West Mexico (slightly different from the others, active diversification of Pinus in particular), Cascades-Sierra Nevada, China, Japan, Taiwan (some Pinaceae in all), and Sabah, the New Guinea Highlands, and New Caledonia (Cupressales dominant).
I do not know of synapomorphies for a clade containing living and fossil conifers (see e.g. Rothwell & Serbet 1994), in part because the extent of the stem group of such a clade is unclear. However, the morphology of extinct conifers and coniferophytes is being re-evaluated as entire organisms are being reconstructed from separate fossil form genera; the result is that many of the conventional taxonomic groupings are being radically overhauled (e.g. Rothwell et al. 2005; Hernandez-Castillo et al. 2009; see also below). As this is done, the extent of the diversity of these fossil plants is becoming clear. Not only are forked leaves common, but stomatal distribution, etc., may differ dramatically on leaves from the one plant, compound microsporangiate strobili are known (c.f. Gnetales!), as are megasporagiate strobili which do not terminate the vegetative growth of the axis on which they occur (e.g. Hernandez-Castillo et al. 2001; Rothwell & Mapes 2001) - but see Cycas...
Diversification in most conifer genera has occurred in the Caenozoic, as emphasized by Klaus et al. (2017), indeed, the median node age of Pinus is a mere 4.4 Ma. Leslie et al. (2012) observed that most southern hemisphere clades are older than northern clades, and this was particularly true of the southern Cupressaceae-Actinostroboideae - its mean node age is four times that of the northern Cupressaceae-Cupressoideae. Leslie et al. (2012) associated this with the more equable climate in at least parts of the southern hemisphere compared with the major climate swings in the northern hemisphere beginning in the Oligocene. Earlier, during the Palaeocene-Eocene thermal maximum, conifers, perhaps especially Cupressaceae and Podocarpaceae, had been replaced by angiosperm-dominated vegetation (Wing & Currano 2013), although now, of course, Pinaceae dominate boreal forests in particular, a change that has happened in the last 12 million years or so, and southern conifers, too, can be locally very abundant.
Extinction rates in conifers in general may have increased during the period 110-100 Ma, i.e. near the beginning of the Cretaceous Terrestrial Revolution, and this is perhaps the result of competition between conifers and angiosperms. These extinction rates have remained high while speciation rates may always have been rather low; the former may be due to more rapid growth, animal pollination, etc., in angiosperms and the latter in part to long generation time, large genome size, etc., in conifers (Condamine et al. 2020 and references). This conifer decline is indeed of long standing, furthermore, the rate of conifer decline may have increased as temperatures decreased in the Oligocene (Condamine et al. 2020). Note, however, as is already clear, competition between conifers and angiosperms can be considered from other points of view, and then conifers may seem to come out not so badly - see Ecology & Physiology below.
The rate of molecular evolution of conifers is substantially slower than that in woody angiosperms, let alone that of herbaceous angiosperms, although the rate of non-synonymous substitutions is higher, perhaps connected with their large populations, outcrossing, and overall low population structure (Buschiazzo et al. 2012; see Jaramillo-Correa et al. 2016; Ran et al. 2018a, also elsewhere under Pinaceae and Araucariaceae). On the other hand, Conifers are unusual compared to other vascular plants in that there has been a gradual increase in their disparity, that is, the total amount/extent of morphological variation in their taxa, since their initial appearance in the Carboniferous (Oyston et al. 2016). More commonly most of the morphospace that a group now occupies comes to be filled very quickly during its initial radiation; for estimates of disparity in major embryophyte groups, see J. W. Clark et al. (2023).
When thinking about the ovuliferous cone, one usually distinguishes between bract and cone scales, the former being subtended by the latter; the whole cone is a compound structure, the ovules being borne on axillary short shoots. However, Herting and Stützel (2020: Fig. 5) suggest that the projection of the funicle beyond the ovule, previously interpreted as a bract or seed scale, is rather a projection of the funicle itself; the funicles and their projections may become laterally fused, as in Pinaceae (= the seed scale), or the ovules may become reduced in number and enveloped by sclerotised (funicular) tissue, as in Araucaria. The orientation of the ovule is often affected, and it ends up being more or less anatropous, i.e. with the micropyle facing the axis, whether because of growth during development of the funicle or not - the latter situation includes Araucaria (Herting & Stützel 2020; see also Herting et al. 2020; Herting & Stützel 2022 - see Bobrov et al. 2025 for possible problems with the interpretation of the tissues of seeds and cone scales in A. angustifolia). In any case, the ovuliferous cone is no longer to be thought of as a compound structure.
Along similar lines, male cones in Pinales and Cupressales (e.g. Araucariaceae, Podocarpaceae, Taxaceae) are also being reinterpreted; they are thought to be pseudanthial in origin, the individual sporophylls being produced by the fusion of several microsporangiophores (see e.g. Schulz et al. 2014; Dörken & Nimsch 2023; Dörken & Stützel 2024; Dörken 2024a, 2024b and references). Thus Dörken (2024a) suggested that teratological pollen cones in Nageia fleuryi were quite possibly evidence of the pseudanthial nature of podocarp pollen cones. In conifers, the microsporangiophores are often hyposporangiate and have two microsporangia that develop on the lower/abaxial side of the stalk and the scutellum is leaf-like; this is the common condition. However, in Araucariaceae and a number of Taxaceae things are different. Thus in Taxaceae there are often perisporangiate microsporangiophores (e.g. Dörken & Stützel 2024), the terminal scutellum is peltate and there are several microsporangia that surround the stalk. Plotting the evolution of the staminate cone on the phylogeny is tricky: A single origin at the base of the conifer tree, and reversals, or...?
Bisexual strobili have ovuliferous scales below the microsporangia, i.e., the same basic arrangement as in angiosperm flowers, and species with such strobili are scattered through the clade (Flores-Rentería et al. 2011). Basic cone morphology is very variable. Conifer seed cones have become more massive and strongly constructed since the Triassic, and particularly the Jurassic, presumably in reponse to animal predation pressure (Leslie 2011b), although Taxus, for instance, is distinctive among extant taxa in having tiny female cones each with a single, erect ovule. The ovuliferous scale is often well-developed and the bract scale inconspicuous, or the bract and ovuliferous scales may be largely separate, as in Pseudotsuga, while in Cupressaceae there is frequently little evidence of an ovuliferous scale in the mature cone, which consists largely of bract scales (e.g. Schulz & Stützel 2007; Rothwell et al. 2011 for references; Aase 1915 for a comprehensive early survey). Understanding details of the morphological evolution of cones will depend on advances in our understanding of the fossil record, and it is likely that heterochrony has been involved; Cupressaceae can be linked with the fossil Voltziaceae (e.g. Rothwell et al. 2011). Developmental studies may also be of value. Thus Englund et al. (2011) found that gene expression patterns linked the epimatium of Podocarpus with the ovuliferous scale of Pinus (see also e.g. Tomlinson & Takaso 2002), but not with the aril of Taxus. However, when comparing the expression of these genes in Cupressaceae, no particular similarities were observable (Groth et al. 2011).
J. A. Doyle (2009) discussed the evolution of exine morphology; granular exine may be an apomophy and then lost twice, or alveolar/honeycomb exine may be plesiomorphous, with granular exine gained three times, or there may be some other combination of gains and losses, but always adding up to three steps. Hence, the acceptance of any particular optimization here is pretty arbitrary.
For differences in the growth of the pollen tube in Pinaceae and angiosperms see Rounds and Bezanilla (2013); branched pollen tubes occur sporadically in Pinales (Friedman 1987 for references). Male gametes need more study. Some taxa have binucleate sperm cells, i.e., a cell plate does not form in the spermatogeneous cell, or, if it does, it is incomplete (e.g. Southworth & Cresti 1997). The male gametes here may be unequal in size, as in Dacrydium, and one may even be extruded from the cytoplasm, as in Podocarpus spp. and Taxus. In at least some Gnetum, Podocarpus andinus, and Torreya taxifolia two unequally-sized male cells are produced (H. Singh 1978 for literature: I am grateful to Ned Friedman for help in understanding this complicated pattern of variation). Double fertlization sometimes occurs in Pinales (Friedman 1992). Southworth and Cresti (1997), Hodges et al. (2011: e.g. Fig. 2A) and others compare the non-ciliated male gametes of Cupressales, Gnetales and Pinales with those of angiosperms. There may be a number of proteins in common with the ciliated male gametes of nearly all other streptophytes, although they do not necessarily have the same functions (Hodges et al. 2011), thus CCP proteins (Coevolved in Ciliated Plants) may have cytoskeletal functions in non-ciliated gametes in the plants just mentioned, things like the microtubule organizing centre, multilayered strucutre, etc., all being absent (see also Southworth & Cresti 1997 for plant microtubules).
At the beginning of embryo development in gymnosperms there is a free-nuclear phase. This free-nuclear stage in the proembryo of Cupressales is shorter than that of most other gymnosperms, there being only 5 or 6 rounds of nuclear divisions in Podocarpaceae and Araucariaceae and even fewer in other members of the order (J. Doyle 1963; Roy Chowdhury 1963; Owens et al. 1995c); the stage is also very short in Pinales (see characterizations). Doyle (1963) followed the development of the immediately subsequent stages, emphasizing the extent of the tiering of cells that develops and the particular tier of cell that gives rise to the elongated suspensor cells. As of 2021 there is no polarity indicated for this variation, some of which is quite striking... The size of the mature embryo is rather variable, although it is often rather larger than that of the common ancestor of extant seed plants; in Pinus it may be close to the length of the seed.
Ecology & Physiology. There may not be that many species of conifers, but they are currently ecologically a remarkably successful group. Single species dominate large areas in the extensive Boreal/Taiga forests in particular, but they are common in many other places outside the humid lowland tropics. Overall, they are more or less dominant in 39% of the world's forests, yet they include a mere 615 species or so, compared with the ca 352,000 species of angiosperms, so they are less than 0.27% of all seed plants (see also Krokene et al. 2008; Ran et al. 2018a, etc., also below). Recent estimates are that there are around 73,000 species of trees, of which perhaps 9,000 are yet to be described (Gatti et al. 2022), but not all the 615 or so species of Pinales and Cupressales are trees; conifers make up less than 1% of all described trees. However, H. Ma et al. (2023) estimated that individuals of all needle-leaved species of trees, i.e. conifers (most of them are evergreen), made up 43% of total trees, a remarkable figure. Although the above-ground biomass of these trees is some 145 Gt, around 24% of the total, so in proportion rather less (but still a pretty remarkable figure), this does not take into account the biomass of these forests that is underground, and this underground biomass is very important (Hicks Pries et al. 2023) - for more on the total carbon sequestration in such forests, see also elsewhere. Ma et al. (2023) noted that the leaf form of trees, i.e. broad- versus needle-leaved, was correlated with temperature, while leaf habit, i.e. deciduous versus evergreen, was more linked to climate and soil.
Bond (1989), Keeley and Zedler (1998), Brodribb et al. (2012), Augusto et al. (2014) and others have emphasized that after the seedling stage conifers in general can out-compete angiosperms in a number of environments that are low in nutrients, conifers being able to tolerate poor soils; they can also handle extreme conditions such as drought and cold (the [Podocarpaceae + Araucariaceae] clade tolerates extreme cold less well), they also grow well in high-light environments, and despite lacking vessels their wood shows moderate hydraulic conductance and is resistant to cavitation, etc. (Pittermann et al. 2010; Brodribb et al. 2012; Augusto et al. 2014). Indeed, their wood has larger hydraulic safety margins than that of angiosperms, so they can handle extreme drought conditions better, although Pinus may be an exception here (Breshears et al. 2005; Stovall et al. 2019). However, Janssen et al. (2012) found that in a number of species plasmodesmatal strands traversed the torus, presumably allowing any cavitations to spread, although the authors could think of no functional value for this.
Sperry (2003), Pittermann et al. (2005, 2011), Hacke et al. (2005, 2015) and Sperry et al. (2006) compare water transport in tracheids that have the torus:margo pits found in many conifers (also including Gnetum and Ephedra, but not cycads or Ginkgo), with that in other kinds of tracheids and in vessels. Pore size in the margo is relatively large facilitating water transport, while the torus provides a valuable safety feature guarding against embolism as it will plug the pit if pushed against one side by pressure from the embolism. Indeed, hydraulic conductance in tracheids with torus:margo pits is somewhat greater than in vessels of similar diameter when expressed on a sapwood area basis, while studies of cavitation in this system suggest that it is not connected with the size of the pores in the margo, but rather with the torus:pit aperture ratio, since if the torus is relatively too narrow, air will seep in around the sides (Pittermann et al. 2010). Interestingly, a few conifers in most families (Podocarpaceae and Araucariaceae not examined) were found to have plasodesmatal pores in the torus which may comprimise the cavitation resistence of the tracheids concerned (Jansen et al. 2012). Vascular tissue with tracheids only may be less hydraulically efficient than vascular tissue with vessels that have simple perforations, but tracheids are tolerant of hydraulic stress and are resistant to cavitation, despite gymnosperms investing only about half as much as angiosperms in wall material - but at the same time they can make up the trunk of very large trees (Sperry 2003; Hacke et al. 2015). Lipid surfactants in the xylem of angiosperms, at least, i.a. coat nanobubbles as they form and so prevent the formation of embolisms, and they should be looked for here, too (Schenk et al. 2017). As Pittermann et al. (2005: p. 1924) note, "the evolution of the torus-margo membrane within the gymnosperm lineage from homogeneous pits was equivalent to the evolution of vessels within the angiosperms" (see also Wilson 2015; Hacke et al. 2015), but water transport can be surprisingly efficient in ferns, too (Pittermann et al. 2011, 2015). To summarize: given the stressful abiotic environment of the boreal forest biome in which conifers dominate, trees with such tracheidal tissue, relatively efficient as to wood construction costs yet "safe" in terms of water conductance (e.g. resistant to cavitation), will be favoured (e.g. Hacke et al. 2005, 2015; Swenson & Enquist 2007). For water - and sugar - movement within a gymnosperm leaf, see elsewhere.
The diameter of first order roots is linked to mycorrhizal type and how the plant forages for nutrients, and it varies considerably (W. Chen et al. 2013, 2016), but no comprehensive survey seems to have been carried out.
Many Pinaceae, ECM plants, grow successfully in low N conditions, while podocarps, AM plants, can grow in low P conditions (Brodribb et al. 2012).
Litter and wood decay of gymnosperms in general is slower than that of angiosperms (e.g. Wardle et al. 2008; Cornwell et al. 2008b; Weedon et al. 2009), and root decay of conifers in particular is slower (Silver & Miya 2001), however, litter of the arbuscular mycorrhizal juniper has a lower C:N ratio than that of Pinus edulis and decomposes faster (Gehring et al. 2017b and references). Brown rot fungi such as boletes, even some ascomycetes, are common on conifer wood, and here lignin is very largely G[guaiacyl]-type; brown-rot fungi with their manganese peroxidases that can break down phenolic compounds digest cellulose and hemicellulose, but not so much lignin itself, although it may get demethylated ("brown" rot refers to the colour of the lignin-rich material remaining after the activities of these fungi). Note that lignin from pteridophytes, other gymnosperms, etc., often includes appreciable if still minor amounts of S-type lignins, and these are more complex, with a lower phenolic content and less degradable, and also H-type lignins, with p-parahydroxyphenyl units; G-type lignin is still normally the major component (see Ayuso-Fernández et al. 2019 for details of lignin decomposition). Brown-rot fungi have often evolved from white-rot fungi (e.g. Floudas et al. 2012; Kohler et al. 2015), although the basal dacrymycete Calcera cornea may have evolved from a soft-rot ancestor (Nagy et al. 2015).
Conifers frequently dominate the communities in which they grow and are often long-lived plants, so ceteris paribus the rate of their evolution will be slow. The high-light conditions they prefer are often associated with infrequent catastrophic disturbances that may kill the adults, but they also allow seedling establishment. Such disturbances are mainly caused by fires, and these are frequently common in communities where conifers dominate, or are at least common, and four adaptations within the conifer clade are paricularly conspicuous here: Thick bark and a seedling "grass" stage (both associated with low-intensity surface fires), and resprouting (for which, see Clarke et al. 2012) and serotiny, both associated with high-intensity crown fires (Turck et al. 2025). Pinales and Cupressales in particular span the spectrum non-flammable—fast-flammable—hot-flammable, the latter being associated with crown fires and the death of the plant, serotiny or at least fire-stimulated germination, while fast-flammable fires occur in more savanna-like communities (Pausas et al. 2017; Stevens et al. 2020). Other features associated with the fire regimes include density of the leaves on the ground (long needles are associated wih lower density of the litter and fast-flammable fires), branch shedding, leaves in dense tufts, ability of the plant to sucker and the like (Pausas et al. 2017; B. Wang et al. 2024). Adaptations to fire are particularly noticeable in species of Pinus subgenus Pinus, a group of southern hemisphere Cupressaceae like Callitris, Widdringtonia, Fitzroya and Actinostrobus, northern Cupressaceae like Hesperocyparis, also Sequoia, Araucaria, the alpine podocarp Halocarpus bidwillii and so on, quite often in Mediterranean climates (Turck et al. 2025).
Thinking of ages repored for conifers, aside from Pinus longaeva (Pinales), which, at some 4,844 years old, is the oldest known non-clonal seed plant, there are records of individuals of five more species, all Cupressales, that lived to over 2,000 years, while a number of other species from both Cupressales and Pinales can live for over 1,000 years - ages that are at least double those of the oldest ages of most angiosperms (Piovesan & Bondi 2021). One can think of growth/survival and stature/recruitment strategies playing off against each other; long-lived conifers tend to be found in nutrient-poor conditions where there is slow growth, and in glacial refugia and places where there has been little effect of humans on the vegetation; the plants also invest heavily in insect, etc., defences, and growth plasticity and modularity are all important (Piovesan & Bondi 2021). In general, fast-growing trees die young!
Some emergent and apparently dominant conifers (often other than Pinaceae, Araucaria is an example) may have have remarkably little effect on the forests in which they grow. In such cases the basal area of angiosperm trees in forests with and without these emergents are similar (Enright & Ogden 1995; Aiba et al. 2007).
Pollination Biology & Seed Dispersal. For pollinators of fossil coniferales, see Peris et al. (2017). Fossils suggest Cheirolepidaceae were visited by Neuroptera ca 155 Ma and by Diptera 130-105 Ma (Peris et al. 2017).
There are three main kinds of reproductive cycles in Pinales and these are based on how long reproduction takes - one (the common condition), two, or three years. In the two-year cycle, either both fertilization and seed development occur in the second year, the pollen tube having stopped growth over the winter, or fertilization may occur in the first year, and it is the immature embryo that overwinters. Both kinds of slow-down occur in the course of the three-year cycle which is known from pines (Turgeon et al. 1994; Owens et al. 2003).
Much has been learned about pollination and pollen germination in conifers in the last few years, and this is summarized by Leslie et al. (2015a), although important work had been carried out about 80 years ago. Details of the fascinating story in Leslie et al. (2015a) depend on the topology of the tree used, which in Podocarpaceae, for instance, differs from that below (in such cases, data checked against Leslie et al. 2015b). Wind pollination is ubiquitous. The pollen grains directly impact the ovulate cones rather than being swept around them by a turbine-like action (Cresswell et al. 2007). There are correlations between the presence of pollen sacci or wings, exine thickness and structure, whether (no wings) or not (wings) the pollen is wettable/pollen buoyancy, ovule and cone orientation, and presence of a pollination drop (Tomlinson 1994; Little et al. 2014; Leslie et al. 2015a). For the composition of the pollination droplet, see Ziegler (1959) and Nepi et al. (2009, esp. 2017); like other wind-pollinated gymnosperms, it is relatively lower in sugars but higher in amino acids than gymnosperms in which insect pollination is sometimes involved.
Recent work suggests that pollen morphology variously affects pollen dispersal, pollination, and the beginning of the fertilization process. Sacci may indeed increase the distance the pollen grain can travel before it falls to the ground, so facilitating wind dispersal (Schwendemann et al. 2007), and this is true of fossils as well, although the shape of some fossil conifers grains is pretty remarkable - Gothania grains look like blimps (Grega et al. 2018). However, the role of sacci in dispersal depends in part on the nature of the sacci; if they have extensive air sacs, as in Pinus, pollen is likely to travel further (as also if the size of the sacci is increased in simulation), but if the sacci are composed of denser material, as in Falcatifolium, the pollen will fall faster (as with increase of saccus size in simulations)- as it may also if the surface of the pollen grain is not smooth (Grega et al. 2013). In some species sacci on pollen function almost like water wings, helping to orient the pollen grains in the pollination droplet (J. Doyle & O'Leary 1935; Salter et al. 2002 and references), or, more particularly, when the ovules are inverted, a common condition, the pollen grains are wetted and float up to the micropyle where the sacci orient the grain on the nucellus, separating and exposing the sulcus through which the pollen tube germinates (Salter et al. 2002; Leslie 2010b); note, however, that Schwendemann et al. (2007) found that when pollen grains landed the sacci, between which the pollen aperture lies, faced upwards. As the pollen grain in flight dries, the sacci move closer together, closing around the aperture (Schwendemann et al. 2007), and this will increase the settling speed (Grega et al. 2013). The surface of the grain is also important - in taxa with high Reynolds numbers surface texture increases the drag, but in those with lower numbers it reduces the drag, pollen grains falling faster (Grega et al. 2013). Sacci also help in the selection of appropriate pollen grains during pollination, thus the proportion of saccate to non-saccate pollen grains inside the ovules is higher than that outside (Leslie 2009). The pollination droplet in Phyllocladus and many taxa with erect ovules is resorbed through the micropyle, and again the pollen grains are brought close to the nucellus; in Juniperus communis and other taxa this resorbtion may be an active process that happens quite soon after the pollen grain lands (Mugnaini et al. 2007). There are further variants of these pollination mechanisms in Pinales (e.g. Owens et al. 1998; Salter et al. 2002; Fernando et al. 2005; Nepi et al. 2017) and in other ancient gymnosperms (Leslie 2008), and the esaccate pollen of may even have been animal dispersed (Grega et al. 2018). The pollination droplet is sweet and may also be involved in insect pollination being taken up by visiting insects, as in a number of Gnetales q.v.. Changes in pollination mechanisms seem not to be accompanied by changes in diversification rates. Although sacci have been lost several times, in no case have sacci re-evolved within esaccate clades, loss being something of a one-way street (Leslie et al. 2015a). For additional information on pollination, see J. Doyle (1945), Niklas (1985: aerodynamics of pollination, including in early Palaeozoic plants); Tomlinson (1994, 2000, 2012), Tomlinson et al. (1997), Tomlinson and Takaso (2002) and Williams (2009), also, work on wind-dispersed spores in all groups is relevant to what goes on here - so see spore dispersal in bryophytes, lycophytes, Gnetales, leptosporangiate ferns, and even wind-pollinated angiosperms under Pollination & Seed Dispersal/Fertilization & Spore Dispersal. .
There is considerable variation in the development of the male gametophyte (Fernando et al. 2010: summary, terms used). The actual process of pollen germination varies, and the feature "pollen exine shed during microgametophyte germination", is likely to have evolved more than once (?three times) in Pinaceae alone (see also Rydin & Friis 2005); for cell death induced by the growing pollen tube, see Fernando et al. (2005 and references). Proteins have been found in the pollination droplet, and these may be involved in defence against pathogens and in promoting male gametophyte development (Wagner et al. 2007). The female gametophyte is monosporic and several archegonia develop, but they are all the same genotype - exceptions are Cupressus sempervivum (?= C. sempervirens) and Gnetum and Welwitschia (Gnetales) (Haig 2020).
For details of seed morphology, dispersal type, etc., and their evolution, see Contreras et al. (2016). Fleshy (the fleshiness arises in several ways) animal-dispersed seeds have evolved several times from winged seeds, and there have been no reversals, however, the dry animal-dispersed seed type in Pinus has reversed to the winged type (Contreras et al. 2016). Bateman et al. (2011; see also Givnish 1980) note a correlation between dioecy and fleshy, animal-dispersed seeds and monoecy and dry, wind-dispersed seeds in gymnosperms, a correlation that is evident within Pinales. Leslie et al. (2013) suggest that this is largely due to the persistence of groups which are dioecious and have fleshy disseminules and monoecious and have dry disseminules; they note common transitions from the monoecy/fleshy to dioecy/fleshy combinations, although overall such features had little effect on diversification. Pollen and dry seed cone size in particular correlates with branch thickness, etc., in conifers; fleshy seed cones are notably smaller than dry cones (Leslie et al. 2014). Variation in seed size links with dispersal mechanisms - abiotic dispersal [small] < seed subtended by attractant tissues < attractant issues part of the seed [large] - and there are also correlations with clades (Leslie & Beaulieu 2015; see also Leslie et al. 2017 for detailed discussion). Furthermore, cone scales may reflex to allow the seeds to disperse, or they may fall from the cone, seeds dispersing at the same time; the latter condition is commoner when the seeds are larger and are packed more densely in the cone (Losada et al. 2019). Herting et al. (2020) discuss details of the morphology of the ancestral conifer seed cone. There is substantial variation in the fatty acid composition of seed oils (Wolff et al. 1997) that may be linkable with phylogeny.
Plant-Animal Interactions. There was an increase in insect damage types in gymnosperm fossils from the Late Triassic to Middle Jurassic, indeed, a number of gymnosperm lineages were diversifying then; by the middle of the Jurassic or thereabouts gymnosperms showed a modern/angiosperm pattern of herbivory with low turnover and high nestedness when sites are compared (L. Xiao et al. 2025). Looking at leaf herbivory in extant vascular plants, Turcotte et al. (2014: see caveats) found that it was relatively low in gymnosperms as a whole. In an earlier survey (Turgeon et al. 1994), almost 400 species of insects were recorded as eating conifer seed cones, and of these almost half (184 spp.) were Lepidoptera. Larger genera involved include Megastigmus (torimyid Hymenoptera), Dioryctria (pyralid moths) and Cydia (tortricid moths). Caterpillars of Yponomeutoidea-Argyresthidae, ditrysian moths, some of them leaf miners, are quite common here (Sohn et al. 2013). Gymnosperm feeders in the “Symphyta”-Tenthredinoidea sawfly group are members of Diprionidae, a small group of some 140 species that diverged from within Tenthredinoidea ca (137-)104(-75) Ma (Isaka & Sato 2015).
Conifers have a variety of defences against insects, both constitutive and induced (e.g. Krokene et al. 2008). Franceschi et al. (2008) note possible anatomical apomorphies that are associated with defence against herbivorous insects. All conifers have layers of polyphenol-containing parenchyma (PP) cells in the phloem, possibly offering some protection against insects (Li et al. 2012), and methyl jasmonate is part of the inductive pathway (e.g. Hudgins et al. 2003; Hudgins & Franceschi 2004); these PP cells contain unidentified polyphenols, and they may also produce more PP cells, wound periderm, new cork cambium (Krokene et al. 2008). Resins are either constitutive or inducible, in the latter case developing in response to stimuli like insect attack, wounding, and the like; perhaps ironically, bark beetles use these resins to produce pheromones that result in the aggregation of the beetles and the overwhelming of the plant's defences (Krokene et al. 2008). In conifers in general, constitutive resin-based defences are found only in some Pinaceae, which, paradoxically, are susceptible to attacks by scolytid beetles, while Cupressales largely have inducible resiniferous structures, or they may lack both constitutive and inducible rein defences, but are not susceptible to attack by these beetles (e.g. Aloni 2021). For details of resin structures, see Krokene et al. (2008), they note i.a. that induced resin canals will tend to be vertical, while constitutive canals can be radial. The pattern of evolution of these resin defences seems rather miscellaneous, but this may be because I have not looked at the pertinent primary literature (but c.f. Franceschi et al. 2005; Krokene et al. 2008; Krokene 2015). There are other defences, too, the PP cells mentioned (most studied in Pinaceae) in the phloem, and arranged in various ways, calcium oxalate crystals of different sizes, lignified tissues of various kinds (Krokene et al. 2008); for further details, see the characterizations of individual groups. Different herbivores elicit different responses by the plant (Moreira et al. 2013), and even within Pinus there is variation in how much particular defences are expressed constitutively, the very distinction between constitutive and inducible sometimes being unclear (Krokene et al. 2008; Carrillo-Gavilán et al. 2014; Krokene 2015). See below for some details about the interactions between bark beetles, their associated fungi, and the conifer host.
All told, about 100 genera of insects prey on conifer mainly Pinaceae and Cupressaceae - seed cones, Megastigmus (Hymenoptera-Torymidae) being a paarticularly notable pest (Roualt et al. 2004).
Conifers are noted hosts of aphids (Aphididae), and some 350 species (out of ca 5,000 total), mostly from not very speciose basal clades, are known to live on the group. Adelgidae, close to Aphididae, are also to be found on conifers, whence they may have moved to angiosperms, and alternation of hosts is quite common in conifer aphids (Peccoud et al. 2010). Favret and Voegtlin (2004), Meseguer et al. (2015) and R. Chen et al. (2016) discuss speciation of Cinara aphids, found on Cupressaceae and especially Pinaceae (see also below).
Plant-Bacterial/Fungal Associations. A number of rusts, including those on ferns, Rosaceae, Grossulariaceae, etc., have part of their life cycles on Pinales, especially Pinaceae (Savile 1979b). For foliar endophytes and their bacterial associates, see Hoffman and Arnold (2010). Basidiomycete brown rot fungi are common in coniferous forests and are also found in broad-leaved, more temperate forests, but they are at most uncommon in tropical forests (Gilbertson 1981); for the relationships of (and uncertain distinction between) brown and white rot fungi, see Riley et al. (2014), Nagy et al. (2015). For the blue-staining ascomycete fungi associated with ambrosia beetles, see below.
Vegetative Variation. Secondary growth (only phloem is produced) has been reported from the leaves of a number of conifers, including those of Pinus longaeva the needles of which can live for 30 years or more (Ewers 1982; Hacke et al. 2015). There are short shoots in a number of taxa (see Dörken 2012 for a summary), and the shedding of branchlets, cladoptosis, which also occurs in taxa other than those with short shoots, is widespread (Burrows et al. 2007 and references; Dörken 2012). Below a distinction is made between evergreen and deciduous trees, the latter having no photosynthetic leaves during the winter, and cladoptosis, the shedding or abscission of branchlets. The latter may be the normal way in which leaves fall from the plant in both evergreen (e.g. Araucaria) and deciduous (e.g. Metasequoia) taxa and in those with (e.g. Pinus - evergreen) and without (e.g. Taxodium - deciduous) short shoots. (Note that the distinction between short and long shoots here is whether (long shoots) or not (short shoots) internodes are elongated.) Organised bud meristems usually occur in the axils of only some leaves, although more cryptic meristems are quite widespread (Namboodiri & Beck 1968a; Fink 1984; Burrows 1999, 2009); in taxa like Pinus and Sciadopitys axillary buds on the main axes are much more common, although most produce short shoots. Serial axillary buds occur in some taxa, particularly in those with cladoptosis since in their absence the supply of reserve meristems along a branch is reduced with the abscission of the branchlets; the oldest buds are furthest away from the axil.
Genes & Genomes. De Miguel et al. (2015) that the ancestor oif [Pinales + Cupressales] had 20 major linkage groups, from which the genomes of Pinales, x = 12, and Cupressales, x = 11, had been derived. However, little is known about genome structure in Cupressales, and thinking of Amborella as an outgroup (de Miguel et al. 2015) may not help very much. Chromosome number shows little variation in Pinales, and overall there is little polyploidy, less than 1/5th that in angiosperms (Murray 2013; Halabi et al. 2023). Based on their study of the coast redwood, Sequoia sempervirens, the only hexaploid in the whole group, Scott et al. (2016) emphasized the rarity of polyploidy in extant conifers, perhaps connected with their slow rate of diploidization. However, there do appear to have been three genome duplications in the conifer area, one in stem Pinaceae, another in stem [Taxaceae + Cupressaceae] (Sciadopitys was not examined), and also a third in Welwitschia (Z. Li et al. 2015; Li & Barker 2019/2020, but c.f. Zwaenepoel & Van de Peer 2019).
Conifers are noted for their very large nuclear genomes with 2 C values of up to 72 pg (Zonneveld 2012); for 1 C values, see the Plant DNA C-values Database (consulted vi.2013). These massive genomes are in part the result of the activity of a number of transposable elements that is not counteracted by mechanisms for slimming genomes, as in angiosperms (Ahuja & Neale 1005; Nystedt et al. 2013; see also Guan et al. 2016: Stevens et al. 2016). Note, however, that genome size in Pinales is quite similar to that in other gymnosperms. There have probably been reductions in genome size in Podocarpaceae and also in particular in Gnetum, but not in Ephedra, despite the prevalence of polyploidy there - it is known from ca 80% of the species for which there was cytological data (Ickert-Bond et al. 2020; see also H. Wu et al. 2020). The increase in genome size in gymnosperms is not (quite) entirely a one-way ticket (c.f. Bennetzen & Kellogg 1997). Leaf mass per unit area seems to be associated with with genome size, but this may be because of phylogenetic correlations (Beaulieu et al. 2007b).
For the inheritance of chloroplasts and mitochondria in conifers, sometimes leaky, see Adams (2019); heteroplasmy is quite widespread (references in C. Lee et al. 2020). Isomeric plastomes are commonly recorded (Lee et al. 2020: ?not in Araucariaceae, Gnetales, Ginkgoales).
C.-S. Wu et al. (2011b) suggested that a different copy of the chloroplast inverted repeat (IR) had been lost in Pinales (the IRb copy) and in Cupressales (the IRa copy). Raubesen and Jansen (1992a), Lackey and Raubeson (2008), Hirao et al. (2008) and Yi et al. (2013: can the copies be distinguished?) also discuss the loss of a copy of the IR; Guo et al. (2014) and J. Li et al (2016) note that in both cases short and novel IRs have developed. Overall, plastome size is small, although c.f. Ginkgo (Y. Yu et al. 2019b).
There was no particular correlation between mtDNA copy number and synonymous substitution rates in gymnosperms, unlike the negative correlation in angiosperms; this may be connected with the longer generation times in those gymnosperms exmained (Zwonitzer et al. 2024).
Chemistry, Morphology, etc.. For fatty acids in the seeds, see Wolff et al. (2002 and references), and for resin composition and gum production, see Tappert et al. (2011). Conifer lignin, primarily made up of guaiacyl units, is denser, more highly condensed, has a larger polymer size, etc., than the S-rich lignins of angiosperms; the two also differ in how common a numebr of minor lignin components are (A. Wagner et al. 2012, 2015). Andersson et al. (1973) briefly mention lignin composition of the bark of some conifers.
Noelle (1910: all Pinales and Cupressales except Podocarpaceae) is a still useful survey of root anatomy, for instance, Gerrath et al. (2002) built on this to discuss the systematic significance of phi thickenings in the root cortex, while Noelle's findings on the presence/absence of root hairs seems to correlate with major groups. The interpretation of the stem apex in terms of tunica-corpus construction is not easy (see Napp-Zinn 1966). I have not integrated much of the considerable variation in wood anatomy with the clades recognised here (see e.g. Zhou & Jiang 1992 for information). Cork cambium is often more or less deep seated, although in Sequoia and Phyllocladus, for example, is is superficial (Möller 1882). Bark anatomy is very complex, but fortunately it has been studied in detail (e.g. Franceschi et al. 2008). Calcium oxalate microcrystals are commonly found in some cell walls throughout the group (Fink 1991; Hudgins et al. 2003: ?Cephalotaxaceae, Sciadopityaceae), but their distribution in other gymnosperms is unclear; they may be absent. Their position within tissues is linked with the development of fibres, the amount of resin secreted, etc. (Hudgins et al. 2003). There is generally a single vascular trace per leaf, but if the leaves are opposite, there may be two traces, but then they fuse before they enter the petiole (Namboodiri & Beck 1968a, b), and leaf traces can also make connections with xylem produced during the second and subsequent years (Maton & Gartner 2005). Which taxa (few? most?) have a foliar endodermis is unclear (c.f. Lersten 1997; Dörken 2014). Dörken (2012) discussed the long/short-shoot distinction, suggesting that shoot differentiation in genera like Pinus and Sciadopitys was a "reminiscence of a deciduous ancestor" (ibid.: p. 81).
Detailed studies on both fossil and extant conifers by Florin (e.g. 1951) laid the foundation for subsequent work on the group; see also Page (1990) and especially Gifford and Foster (1988) and Farjon (2005b: bibliography), also Farjon (2008) for an excellent general account; see also Debreczy and Rácz (2006), Eckenwalder (2009), Garnandt et al. (2011) and Plomion et al. (2011) for general information, Trapp and Croteau (2001a: resin biosynthesis), IAWA Committee (2004: features of wood anatomy used in identification, and more), Zhou and Jiang (1992: wood anatomy), Geyler (1867), Barthelmess 1935, and Kumari (1963: nodal anatomy), Möller (1882: cork cambium), Napp-Zinn (1966) and Yao and Hu (1982), both leaf anatomy, Den Outer (1967) and Schulz (1990), both phloem anatomy, much detail unincorporated, Khan et al. (2019: epidermis); see Mundry (2000: cone/strobilus development, emphasis on Taxaceae and friends), also Konar and Oberoi (1960) and Williams (2009), both reproductive biology, Sivak (1975: detailed study of saccate pollen), Owens et al. (1995b: cytoplasmic inheritance, nuclei sometimes incorporate cytoplasm), Sklonnaya and Ruguzova (2003: spermatogenesis), Bobrov and Melikian (2006: seed anatomy, both testa and tegmen present?), Buchholz (1929: embryogeny), Butts and Buchholz (1940: cotyledon number), Hill and de Fraine (1908, 1909: seedlings), Mathews and Tremonte (2012: greening of seedlings in the dark), and Herrmann (1951: intergeneric grafting). A valuable resource is the Gymnosperm Database (Earle 1997 onwards).
For further information on the major seed plant groups, see angiosperms, Cupressales (below), Cycadales, Ginkgoales, Gnetales and Pinales, and for discussion about their relationships, see Angiosperm History I, also above for conifers in general, and elsewhere for the particular problem of Gnetales.
Within conifers, relationships are being clarified. Pinaceae (Pinus, Cedrus, etc.) are sister to the rest, as a morphological cladistic analysis by Hart (1987) suggested some time ago (but c.f. Nixon et al. 1994; J. A. Doyle 1998b). Molecular data and additional morphological work largely confirm the relationships in the tree here, which is based on the work of Quinn et al. (2002: successive approximations weighting), see also Price et al. (1993), Tsumura et al. (1995: RFLP analysis, tree [unrooted] with the same topology as the tree above), Kelch and Cranfill (2000), Gugerli et al. (2001: e.g. the mitochondrial nadI gene), Rai et al. (2002, especially 2008a), Burleigh et al. (2012), the four-gene analysis of Leslie et al. (2012) with its excellent sampling (but not Gnetum, etc.) and Ruhfel et al. (2014). However, the topologies in the scenarios of Biffin et al. (2010b) are either [Pinaceae [Podocarpaceae + Araucariaceae]] or [Pinaceae + Sciadopityaceae, etc.]... Sciadopityaceae are also somewhat migratory in the nuclear gene analyses of Y. Lu et al (2014), being either sister to [Taxaceae + Cephalotaxaceae] or to [Podocarpaceae + Araucariaceae] depending on whether the 1st and 2nd codon positions alone were included, or all three.
Gnetales were not included in the studies cited in the previous paragraph. Indeed, the position of Gnetales has long been problematical, and this is discussed elsewhere. Ignoring Gnetales, relationships in the conifers can be depicted as follows.
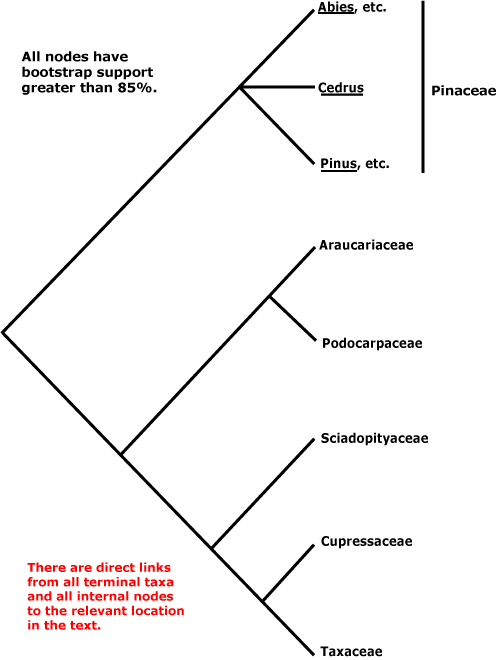
Classification. Producing evolutionary classifications, or classifications that emphasised one or two favoured morphological characters, remained popular for quite some time among those working on conifers (e.g. Keng 1975; Melikian & Bobrov 2000; Fu et al. 2004: Nageiaceae and Podocarpaceae well separated; Bobrov & Melikian 2006: Araucariaceae and other conifers quite separate from Pinaceae and Sciadopityaceae). However, see Farjon (1990, 2005a, c, 2017) in particular for detailed treatments of the conifers, Farjon (2001) for a checklist, and Christenhusz et al. (2011b) for a linear classification.
CUPRESSALES Link / Conifers II / Cupressophyta - Main Tree
Highly oxygenated diterpenes with phenolic rings [phenolic abietanes]; root hairs 0[?]; reaction wood on lower side of stem; resin canals 0; vessels 0, phloem with ring of parenchyma cells containing phenolic compounds one cell across, alternating with ring of phloem, ring of fibre cells with crystals in walls, ring of phloem, then ring of PP cells again, etc., calcium oxalate crystals small, extracellular; (leaves opposite, sometimes then with two vascular traces); male gametophyte: pollen grains atectate, after pollination only male gametes produced [?here]; early embryogeny: initially with 2 to 5(-6) free-nuclear divisions, nuclei not in any particular arrangement, then basal embryonal cells not particularly organized, a tier of elongated suspensor cells, and upper tier of nuclei, partly or completely surrounded by walls; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], chloroplast IRa copy lost, but ycf2 and psbA regions retained, plastid accD protein elongated because of tandem repeats; mitochondrial nadI gene intron 2 lost, also both rps3 introns, duplication in the PHYN clade. - 5 families, 57 genera, 383 species.
Includes Araucariaceae, Cupressaceae, Podocarpaceae, Sciadopityaceae, Taxaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. C. N. Miller (1977) suggests ages for clades here, stem-group Podocarpaceae, at ca 220 Ma in the early Triassic, being the oldest, while crown-group Taxaceae, Araucariaceae and Cupressaceae were dated to the mid-Jurassic, at around 162 Ma. Leslie et al. (2018) suggested an age of (301.7-)278.2(-250.3) Ma for this node, Magallón et al. (2013) an age of around (276.6-)259-256.9(-244.4) Ma and Won and Renner (2006) an age of (303-)273(-243) Ma, while around 249 Ma is the estimate in Ran et al. (2018a) and (282.6-)177.5(-89.1) Ma in Ran et al. (2018b: sampling), 230.9 Ma in Tank et al. (2015: Table S2), as little as 184 Ma in Evkaikina et al. (2017), 361-205 Ma in Herting et al. (2020) and (299.2-)251.9(-201.4) Ma in Ji et al (2020).
Evolution: Divergence & Distribution. Leslie et al. (2018) evaluate the fossil record of Cupressales, and although there is fairly good congruence between the fossil record and the dates they suggest in Cupressaceae, this is not true for Araucariaceae. The fossil record of Podocarpaceae has been evaluated by Andruchow-Colombo et al. (2023b).
Sundaman et al. (2019) listed eight biodiversity hotspots for conifers in general, and these include West Mexico, the Cascades to Sierra Nevada, China, Japan, Taiwan, Sabah, the New Guinea Highlands and New Caledonia. In the last six hotspots in particular, Cupressales are notable components, indeed, in the last three in particular they make up (almost) all the conifers there.
For other possible synapomorphies of this group, see Hart (1987). Isoflavonoids are known from Cupressaceae, Podocarpaceae and Araucariaceae (Reynaud et al. 2005).
Ecology & Physiology. Plants at this node are distinctive in having low leaf nitrogen, extant gymnosperms as a whole having a relatively high ratio of leaf mass per area (Cornwell et al. 2014).
Pollination Biology & Seed Dispersal. The combination of dry disseminules and dioecy has evolved several times in members of this clade growing in the southern hemisphere (Leslie et al. 2013).
Genes & Genomes. Yi et al. (2013) note that the chloroplast accD gene is notably large here (see also Hirao et al. 2008), usually over 600 amino acids long, having become about double the size of that of most other gymnosperms because of tandem repeats that may be unique to particular groups of Cupressales (Sudianto & Chaw 2019). The small repeats may be long enough to allow homologous recombination (for plastome recombination - plastomic sublimons - see Sudianto et al. 2018, ?not in Taxaceae); overall, variation in the chloroplast genome is considerable (e.g. C.-S. Wu et al. 2011b; Wu & Chaw 2014, 2016; Sudianto et al. 2018). J. Li et al. (2016; see also W. Guo et al. 2014) discuss the evolution of the small trnQ inverted repeat in this clade; they suggest as a sequence: single copy (Podocarpaceae, Araucariaceae) → tandem repeat (Sciadopitys) → inverted repeat (the rest).
For mitochondrial genes, especially the rps3 gene, see Ran et al. (2010). For the inheritance of chloroplasts and mitochondria, see Adams (2019 and references).
Chemistry, Morphology, etc.. For southern conifers, making up part of this clade, see Hill and Brodribb (1998: general) and Cox et al. (2007: oxygenated di- and tricyclic terpenoids). Krokene et al. (2008) discuss resiniferous tissues in xylem and phloem and Esteban et al. (2023) provide a useful outline of variation in wood anatomy, terms following those iproposed by the IAWA Committee (2004). Yao and Hu (1982) and Dörken and Stützel (2012a) discuss leaf anatomy; see Sahni (1920) for thickening of tracheidal cells, Khan et al. (2019) discus epidermal features and Roy Chowdhury (1963) embryogeny.
Phylogeny. For relationships, see especially Cycadales and Pinales, also angiosperms, Gnetales and Ginkgoales.
Synonymy: Actinostrobales Doweld, Araucariales Gorozhankin, Athrotaxidales Doweld, Cephalotaxales Reveal, Cunninghamiales Doweld, Cupressales Bromhead, Falcatifoliales Melikian & Bobrov, Metaxyales Doweld, Microstrobales Doweld & Reveal, Parasitaxales Melikian & Bobrov, Podocarpales Reveal, Saxegothaeales Doweld & Reveal, Sciadopityales Reveal, Taxales Knobloch, Taxodiales Heintze - Araucariidae Doweld, Cupressidae Doweld, Podocarpidae Doweld & Reveal, Taxidae Reveal - Araucariopsida A. V. C. F. Bobrov & Melikian, Podocarpopsida Doweld & Reveal, Taxopsida Lotsy
[Araucariaceae + Podocarpaceae]: gums +; roots with beaded nodules; (root suckers +); lamina vascular bundles not surrounded by sheath; three-year reproductive cycle, fertilization in 3rd year - ?); male gametophyte: >6-nucleate - 2 prothallial cells, divide/tube cell/sterile cell/gametes; female gametophyte: ovule one/bract scale, third bulge on the seed scale of Araucariaceae equivalent to bulge on the epimatium of podocarps; seed dispersed as unit with all its supporting structures; proembryo with 5 or 6 free-nuclear divisions [32/64 nuclei]; chondrome paternally inherited; 2nd intron in nad1 gene lost.
Age. The age of this node has been estimated at (287-)257(-228) Ma (Won & Renner 2006), (274.0-)242.8(-197.9) Ma (Leslie et al. 2018), (318-)263(-223) and (255-)198, 177(-157) Ma (Biffin et al. 2010b: latter age preferred), (237-)205(-177) Ma (Biffin et al. 2011b: text, c.f. fig. 2), 230-176 Ma (Leslie et al. 2012), around 243 Ma (Magallón et al. 2013), (284-)250, 225(-202) Ma (Kranitz et al. 2014), ca 186.3 Ma (Y. Lu et al. 2014), ca 195 Ma (Ran et al. 2018a) or 299-175 Ma by Herting et al. (2020).
The fossil record of Podocarpaceae has been evaluated by Andruchow-Colombo et al. (2023b), and the earliest fossils reliably associated with the family are from both hemispheres and are Jurassic in age; fossils of Araucariaceae are also well known from the Jurassic (Leslie et al. 2018).
Evolution: Ecology & Physiology. There are often root nodules in Podocarpaceae and Araucariaceae; those of the former are best studied (see Spratt 1913 for an early survey). The roots involved tend to be superficial in the soil, and the nodules themselves may be in longitudinal rows and represent modified lateral roots (Janse 1897; Becking 1965; Duhoux et al. 2001), although L.-Q. Zhou et al. (2022) and others have suggested that they are not modified roots. Certainly, the anatomy of the mature nodule (see below) is very unlike that of a root. The fungus Glomus may be involved, but nitrogen does not seem to be fixed (Russell et al. 2002), however, Spratt (1913) thought that a "Pseudomonas radicicola" like that in legumes, etc., occurred in the nodules, entering via the roots, and that N was fixed in culture - a bit improbable. Borken et al. (2016) suggested that Lepidothamnus fonkii, which grows in N-poor Patagonian Sphagnum bogs, became associated with Beijerinckiaceae (Hyphomicrobiales), normally free-living N-fixing methanogens; these bacteria grew in the dead outer cells of the nodules, N moving in to the plant. L.-Q. Zhu et al. (2022) thought that the nodules of Podocarpus macrophyllus had features of those of both legumes and non-legumes, indeed, that legume nodules were derived from such Podocarpus nodules. "Zoogleal hyphae" burrowed through the epidermal cells of the host; bacteria entered through these hyphae. There were cysts containing bacteriophages, and the inner cortex contained rhizobia, while in the outer cortex there were vesicles and actinomycetes (Zhu et al. 2022)... Janse (1897: Pl. 7, 8) described fungus-infected nodules from Javanese podocarps (P. cupressinus in particular), and he found that other nodules might develop from these nodules, although not more than five in a row; interestingly, he described long (or just longer?) roots as having similar intermittent growth. Based on Janse's work, particularly that on the longer roots that he illustrated in Pl. 8, Kaplan (2022: p. 1238) suggested that podocarp roots had determinate lateral growth, the axillary branches that developed were more or less terminal, growing at the end of the preceding root, the process repeating itself. In any event, the distinctive beaded nodules that develop in podocarps and Araucariaceae lack a root cap or apical meristem, but they are surrounded by an endodermis, and they develop in two lines opposite the protoxylem poles - the roots are diarch (Schwendemann et al. 2011). Importantly, microorganisms, e.g. fungi, are not needed for the development of these nodules, although fungi move in to them, indeed, their spherical shape would seem to optimise the volume of tissues suitable for associated fungi (Khan & Valder 1972; Dickie & Holdaway 2011; Russell et al. 2002). There are both mycorrhizae and root hairs in some species, which an odd combination; thus the long roots of Prumnopitys ferruginea have root hairs, and such hairs are also found on the nodules of Phyllocladus alpinum (Dickie & Holdaway 2011) and also the nodules of Prumnopitys taxifolia and P. ferruginea (Russell et al. 2002; see also Spratt 1913 for roots, nodules and hairs). Schwendemann et al. (2011) described similar nodules from roots of Notophytum krauselii, a podocarpaceous plant found in Middle Triassic deposits ca 240 Ma from Antarctica. Somewhat confusing the issue, beaded roots, i.e. with continuous central vascular tissue, are known from some podocarps (Dickie & Holdaway 2011). Nodules similar down to anatomical details are found in Araucariaceae, and again, their development is independent of the presence of fungi; these nodules may be terminal or lateral (McGee et al. 1999). Interestingly, McGee et al. (1999: Fig. 6) illustrate terminal conical nodules growing sequentially, and these are at least superficially similar to the elongated structures discussed above in Podocarpus (Janse 1897/Kaplan 2022). All in all, given the uncertainty over the basic root morphology of podocarps and Araucariaceae and that any ecological role these nodules might play is poorly understood (McGee et al. 1999; Russell et al. 2002; Dickie & Holdaway 2011), anatomical/developmental work on this system (with good taxonomic sampling) to supplement ecological studies would be of considerable interest.
Plant-Animal Interactions. Neophyllaphidinae, a basal aphid group and Gondwanan in distribution, are associated with Podocarpus and Araucaria (Peccoud et al. 2010).
Chemistry, Morphology, etc.. For beaded root nodules and their like, see above. Chamberlain (1935) notes that there is no stalk cell per se in the male gametophyte, but when the generative cell divides, one of the resultant cells dies while the remaining cell divides and produces the two gametes. The revised interpretation of the ovuliferous scale, etc., above leads to an equivalence/homology being drawn between the third bulge on the so-called seed scale of Araucariaceae and a bulge on the epimatium of podocarps (see Herting & Stützel 2020: Fig. 4).
ARAUCARIACEAE Henkel & W. Hochstetter - Back to Cupressales
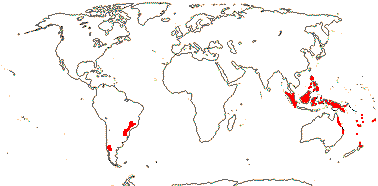
Root cortical cell walls with phi [φ] thickenings; tracheid pitting alternate, polygonal, margo only, phloem sclereids with crystals in their walls [?level], fibre rows 0; branches whorled, plagiotropic, branchlets ultimately abscise [cladoptosis]; secretory cells in the centre of the root; stem apex with tunica/corpus construction; phloem fibres scattered; only resin plugs present in vascular tissue; pits on radial walls of tracheids 1-3-seriate, touching, hexagonal in outline, Bars of Sanio/crassulae thickenings 0; single leaf trace branching profusely in the cortex; axillary meristems on the trunk, undifferentiated, submerged by cork, persistent; stomata tetracytic, (transversely oriented); mucilage cells in foliar parenchyma; leaves multiveined (not), lamina (veins branching dichotomously at base); (plants dioecious); to 20 microsporangia per microsporophyll in parallel rows towards the abaxial side of the stalk; pollen not saccate, pollination drop 0 [?level]; ovule erect, nucellus protrudes from micropyle [stigma-like structure]; male gametophyte: pollen germinates on ovuliferous scale and tubes grow over the scales [extra-ovular capture and germination], tube branched when in nucellus, prothallial cells numerous, gametes engulf cytoplasm of generative cell; seeds developing in association with the bract scale, scales falling from cone at maturity; young embryo central, basally with anticlinally elongated cap cells that degenerate, suspensors several; cotyledons with (3-)4-8 vascular bundles [?Agathis]; n = x = 13, nuclear genome [1 C] 13.5-22.5 pg; (germination cryptocotylar).
3/37: [list]. Southern South America, Sumatra to West Pacific, inc. New Caledonia. Map: from Florin (1963) and de Laubenfels (1988); Cretaceous and Jurassic fossils, green, from Sequiera and Farrell (2001). [Photos - Collection.]
Age. Crown-group Araucariaceae may be 185-165 Ma - or perhaps around 167.2 Ma (Laenen et al. 2014) or 205 Ma (see also Stöckler et al. 2002; Wallis & Trewick 2009). Biffin et al. (2010b) suggest ages of (215-)191(-169) or (94-)65(-47) Ma, the latter being their preferred age; 225-185 Ma is the estimate in Knapp et al. (2007), (179.7-)170.4(-165.2) Ma in Leslie et al. (2018), 172-162 Ma in Wilf and Escapa (2014, q.v. for other dates), (134-)94, 81(-60) Ma in Kranitz et al. (2014), ca 51.2 Ma in Y. Lu et al. (2014), 112-22 Ma in Herting et al. (2020) - or even ca 36 Ma (Crisp & Cook 2011). Dating here is in more than its normal mess.
Araucariaceae are well known as fossils from the Mid Jurassic (ca 175 Ma) onwards, but fossils of the family may go back to the Triassic (e.g. Leslie et al. 2018).
1. Araucaria Jussieu
Phloem with clusters of stone cells, inducible resiniferous structures; bract and ovuliferous scales separate; ligule +, surrounding ovule; tapetum amoeboid; cotyledons (4).
1/19. New Caledonia (14 spp.) to N.E. Australia and New Guinea, South America (southern Brazil southwards).
Age. Crown-group Araucaria is (162.7-)145.4(-127.8) Ma (Leslie et al. 2018).
Araucaria is known from Triassic deposits in many parts of the world in both hemispheres and in both North and South America - for the latter, see A. violetae from early Cretaceous northeast Brazil - it is placed in section Eutacta, extant species of which are known only from the Old World (Batista et al. 2021). The remarkably preserved A. mirabilis in Patagonian middle Jurassic deposits ca 160 Ma has been associated with the monotypic section Bunya (Florin 1963; Stockey 1982, 1994; Hill & Brodribb 1989; Kunzmann 2007; see also Escapa et al. 2018; Hill et al. 2019). However, identification of Araucarioxylon wood can be difficult (Ash & Creber 2000).
2. Agathis Salisbury + Wollemia W. G. Jones, K. Hill & J. M. Allen
(Mucilaginous cells 0 - Agathis); primary branches long, unbranched, short-lived [Wollemia]; leaves opposite/subopposite, (± petiolate), (twisted at base - W.), bract and ovuliferous scales fused; seeds (2-4-ranked when young - W.), winged, wings derived from integument, entire or (asymmetrically) bilobed.
2/18: Agathis (17). Sumatra to Fiji, New Zealand and E. Australia.
Age. This clade may be some (68.6-)57.6(-52.3) Ma (Leslie et al. 2018), a mere (37-)18(-younger) Ma (Crisp & Cook 2011) to at least 110 Ma (Kunzmann 2007; see also Slodownik et al. 2023).
For Agathis fossils, see Hill (2008); the crown-group position of the ca 52.2 Ma South American A. zamunerae (Wilf et al. 2014, see Escapa et al. 2018) is in conflict with some of the ages above. Fossil wood from the end-Oligocene ca 23 Ma in Hainan has been described as A. ledongensis; it is the first record of Agathis from the northern hemisphere, and it probably moved there from Australia as that continent approached southeast Asia (Oskolski et al. 2020).
Evolution: Divergence & Distribution. See also Kranitz et al. (2014) for more ages. Biffin et al. (2010b, esp. 2011b) noted that stem-group calibration scenarios make crown-group divergence of Araucariaceae largely a (mid-Cretaceous to) Cenozoic phenomenon (see also Crisp & Cook 2011). This would question the placement of early fossils in extant sections of Araucaria; indeed, in morphological analyses they form a basal polytomy along with A. hunsteinii and A. bidwillii (Escapa et al. 2018: support low; see also Kranitz et al. 2014). However, there does seem to have been relatively little change in cone and seed scale morphology in sections like Eutacta for a very long time (van der Ham et al. 2010).
Araucariaceae currently have a largely austral distribution, but this is likely to reflect Tertiary events, perhaps being facilitated by movement between South America, Antarctica and Australia. Araucariaceae, along with Podocarpaceae, are the commonest gymnosperm fossils in Antarctica (de la Estrella et al. 2019b: Nothofagus is the commonest angiosperm). Eocene araucariaceous fossils are well known from Patagonia (Markhofer et al. 2015). Although Araucaria is diverse on New Caledonia, there is little genetic divergence between the species on the island, suggesting that the genus has not been there long (Poncet et al. 2019; Gaudeul et al. 2012; Cruaud et al. 2012c; Kranitz et al. 2014). Araucarioid wood was by far the commonest conifer wood in the Barremian-Hauterivian 145.5-125 Ma, but occurrences declined steadily through the Cretaceous, there being few records of any conifer group from the Campanian-Maastrichtian 84-65.5 Ma (Peralta-Medina & Falcon-Lang 2012: Gnetales not mentioned). The current southern distribution of Araucaria is best interpreted as the remnants of a much more widespread range (Stockey 1982; Hill & Brodribb 1989; Kunzmann 2007; Givnish & Renner 2004; Kooymnan et al. 2014; Kranitz et al. 2014; Escapa et al. 2018 for discussion), fossil seeds being known from Europe as recently as the very Late Cretaceous ca 65.7 Ma just before the K/P boundary (van der Ham et al. 2010). These seeds are very similar to those of the extant Australian A. heterophylla (sect. Eutacta), and lower Jurassic seed cone scales have also been compared with those of A. heterophylla (see van der Ham et al. 2010). Interestingly, fossil amber from Myanmar ca 99 Ma seems to be largely derived from Agathis, as does Eocene Baltic amber ca 40 Ma (Poinar 2022), again, these records suggest a much wider past distribution.
Knapp et al. (2007; see also Kranitz et al. 2014) discuss the possibility of the long-term persistence of Agathis in New Zealand since the Eocene or before. Many floristic elements in the island that are known fossil there in the Oligocene-Miocene subsequently seem to have gone extinct, their contemporary representatives being relatively recent immigrants (e.g. Jordan et al. 2010; Puente-Lelièvre et al. 2012), however, the distinctive frog Leiopelma may represent an ancient lineage that has persisted on the islands (Carr et al. 2015). Agathis was until recently thought to be Australian, but well-preserved and abundant fossils have turned up in Patagonian Eocene deposits about 52.2 My; they were previously identified as Zamia (Wilf et al. 2014); see elsewhere for other examples. Araucaria section Eutacta may also fit here, as may Wollemia. Dilwynites pollen is similar to that of Wollemia and some species of Agathis (so it could be the stem group of these genera); it is widely distributed in austral areas, its first records being from the Late Cretaceous up to 93.9 Ma (Mcphail et al. 2013).
The quite recent (1994) discovery very close to Sydney of a few trees of the remarkable Wollemia, very similar to some fossil Araucariaceae (Jones et al. 1995; see e.g. Pastoriza-Piñol 2007 for a general account), occasioned some excitement. However, suggested ages of divergence of Wollemia from Agathis vary considerably as noted above, anything from at least 110-18(-younger) Ma, so some of these ages mean that comparison of Wollemia (and Agathis) with Cretaceous fossils is inappropriate (c.f. Chambers et al. 1998). Also relevant in this context is Araucarioides linearis, a member of a clade sister to [Agathis + Wollemia] and described from Tasmanian deposits 53-50 Ma, the clade being known fron Campanian New Zealand (Slodownik et al. 2023).
For possible apomorphies, perhaps including "dehiscent" seeds, i.e. seeds separating from the cone-scale, see Cantrill and Raine (2006), Escapa and Catalano (2013: most characters quantitative). Apomorphies for the family may be affected by the position of Wollemia within it. Dörken (2024b) suggests that the male cones of Araucaria araucana and Wollemia nobilis, apparently made up of hyposporangiate microsporangiophores, are in fact complex synangial structures of pseudanthial origin - perhaps a feature of conifers as a whole.
Ecology & Physiology. Associations between both Araucaria and Agathis and a variety of insects and fungi may be of very long standing - 50 million years or more. See Plant-Animal Interactions below.
Araucaria, and to a lesser extent Agathis (this is known in amber from Myanmar ca 98 Ma), have been major sources of fossilized resins, a.k.a. amber, since the late Triassic (Seyfullah et al. 2018); Poinar 2022.
For root nodules, see above.
Pollination Biology & Seed Dispersal. The time from pollination to fertilization in Agathis australis is about twelve months, although this includes three months after pollination before the pollen grain germinates, and then another three months over winter when nothing much happens (Owens et al. 1995b). The pollen grains do not rupture when placed in water (Tomlinson 1994).
Seeds in Araucariaceae may be either dessication-tolerant or -sensitive (e.g. Dickie & Pritchard 2002). For the interaction between masting and seed dispersal in Araucaria araucana and the Austral parakeet Enicognathus ferrugineus, which can fly quite long distances, see Sanguinetti and Kitzberger (2008).
Plant-Animal Interactions. Sequeira and Farrell (2001) suggested that the association between Araucaria and the scolytine Hylurgini (used to be Tomicini) bark beetles was Cretaceous in age; the beetles seem to have moved on to Araucaria from angiosperms, and from thence moved on to Pinaceae. These weevils are in a clade separate from the Hylurgini found on pines (Jordal & Cognato 2012). García Massini et al. (2011) found evidence of wood-boring beetles, fungi and mites in fossilized araucarian wood of Middle Jurassic age from Argentinian Patagonia.
Caterpillars of the two species of Agathiphagus (Agathiphagidae), a small group of near-basal jawed lepidoptera, eat the seeds of Agathis, and they are known from Australia to the Pacific (Shields 1988; Powell et al. 1998); the larvae of these moths can remain in diapause for up to twelve years. It has been suggested that Agathiphagidae diverged from the Nothofagus-eating Heterobathmidae as much as 158.5 Ma (Wahlberg et al. 2013), but some favour a position as sister to Micropterigoidea, together forming the basalmost branch of the lepidopteran clade (Regier et al. 2015; Kristiansen et al. 2015; see also Mitter et al. 2016), or as sister to all Lepidoptera apart from Micropterigoidea and (297-)280.6(-257.4) Ma (Kawahara et al. 2019). It would be interesting to learn more about the history of this association.
There are other suggestions of very long-term associations in Araucariaceae. Donovan et al. (2020) looked at the patterns of damage caused by insects - leaf miners, gallers, armoured scale insects and the like - and fungi evident on the fossil Agathis from Patagonian Argentina (see Age above) and found that they were remarkably similar to those on extant Agathis. They suggested that these associations had persisted since the Palaeocene-Eocene even although Agathis is now found thousands of kilometres away from the fossil localities; they acknowledge the possibility that this assembly of organisms may have arisen independently where it now grows (Donovan et al. 2020).
Hummel et al. (2008) suggested that Araucaria, although rather low in protein, was widespread in the Jurassic and was an important component of sauropod diets.
Plant-Bacterial/Fungal Associations. For a possible taxol-producing endophyte, see Strobel et al. (1997). There is discussion about the fungi associated with the roots of Araucariaceae and the nodules there below.
Genes & Genomes. Rates of genome change in Araucariaceae in particular are very low when compared with those of all other land plants (Puttick et al. 2015). However, rates of genome change are low in gymnosperms in general, as are speciation rates, the two being correlated.
Araucariaceae have the largest plastid genomes (ca 145 kb) in gymnosperms but with the greatest percentage (>41.5%) of non-coding content, and in the latter feature they are in line with seed plants in general, except Pinales and Gnetales (C.-S. Wu & Chaw 2016: check).
Chemistry, Morphology, etc.. For the essential oils of Wollemia, see Staniek et al. (2010 and references) amd for a summary of the chemistry of the whole group, see Frazza et l. (2020).
Tomlinson (2008) noted that the axillary branches of Wollemia are evident in the resting terminal bud, but do not grow out until extension growth of the latter starts - a version of syllepsis, perhaps; there are undifferentiated resting meristems in the axils of the leaves of the main axis which develop if the apical meristem is destroyed (Tomlinson & Huggett (2011). The single leaf trace divides into three or more as it proceeds into the leaf (Tomlinson 2008; Tomlinson & Murch 2009), and this may be connected with branch shedding/cladoptosis, which is particularly striking here (Looy 2013 and references). Araucariaceae also have platelet structures in their cuticular waxes (Wilhelmi & Barthlott 1997); the stomata of Araucaria have a wax plug which may block penetration of fungal hyphae (Mohammadian et al. 2009 - see also Winteraceae). Horizontally-oriented stomata are scattered in Araucariaceae, while in Wollemia the stomatal orientation even changes as the plant gets older (Rudall 2023b).
The adaxial part of the cone scale is made up of bract-scale tissues, the adaxial part of ovuliferous scale tissues (Dörken & Rudall 2018). Cones of Araucaria have a "ligule" that is more or less adnate to the ovule. The seeds of Wollemia have integumentary wings, unlike the seeds of other members of the family (Contreras et al. 2016).
For general information, see Stockey (1982), Page (1990), Bieleski and Wilcox (2009), Gee and Tidwell (2010: fossil records from late Triassic to end Cretaceous) and especially the Gymnosperm Database; for comparative anatomy, see Thomson (1913), for axillary buds, see Burrows (1999 and references, 2009), for details of reproductive biology compared with those of other Pinales, see Owens et al. (1995a, b, c), for pollen morphology, see Dettmann and Jarzen (2000), and for the developing embryo, see Roy Chowdhury (1963).
Phylogeny. For a comprehensive phylogeny of Araucariaceae, see Escapa and Catalano (2013). Wollemia has been placed variously sister to Agathis or sister to the rest of the family (Jones et al. 1995; Gilmore & Hill 1997; Setoguchi et al. 1998; S. S. Renner in Kunzmann 2007), the particular position being sensitive to the choice of outgroups (Knapp et al. 2007), but the former position seems more likely. A [Wollemia + Agathis] clade was retrieved in the comprehensive four-gene tree in Leslie et al. (2012) and by Escapa and Catalano (2013). Within Agathis, the New Zealand endemic Agathis australis is sister to the rest of the genus (e.g. Escapa et al. 2018 and references; Leslie et al. 2018). For relationships in Araucaria, see Leslie et al. (2018); the basal clades have strong support.
Botanical Trivia. Araucaria columnaris, the New Caledonian endemic Cook pine, leans towards the south in the northern hemisphere and to the north in the southern hemisphere, and the higher the latitude, the more pronounced the lean (Johns et al. 2017) - tracking the source of solar energy?
Leaves of Araucaria may remain on the plant for 25 years or so (references in Chabot & Hicks 1982; Lusk 2001), perhaps #3 behind Welwitschia and Pinus.
PODOCARPACEAE Endlicher - Back to Cupressales
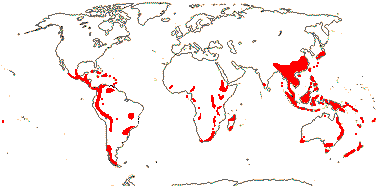
Podocarpic acid + [diterpene with phenolic ring], (syringyl lignin + - positive Mäule reaction); tracheid pits with margo + torus/(margo alone/0); (nodes 1:2); sclereids numerous, with large lumen; leaves scale-like, blade with accessory transfusion tissue in patches perpendicular to vascular bundles/0, laterally-elongated sclereids in middle of blade; plant dioecious; microsporophylls with two sporangia; pollen exine thin, except distally; male gametophyte: prothallial cells 3-6(-8), nuclei of male gametes unequal in size, (one extruded); proembryo cells binucleate (only one such cell) [≡ E tier cells]; 1 seed/cone, associated with ± fleshy structures [ovuliferous scale = epimatium, receptacle]; polyembryony common; cotyledon with two vascular bundles [?all]; nuclear genome [1 C] 4-11(-13.8 pg - Manaoa colensoi).
17/186: [list, to subfamilies]. Largely Southern Hemisphere, scattered, N. to Japan, Central America and the Caribbean. Map: from Florin (1963), Dalling et al. (2011) and Adie et al. (2011).
Age. Crown-group Podocarpaceae are estimated to be ca 102 Ma (Magallón et al. 2013), although many other estimates are substantially older, ca 132.7 Ma (Y. Lu et al. 2014), (194-)145(-99) Ma in Biffin et al. (2011b: c.f. Fig. 2), (223.6-)188.9(-159.5) Ma (Leslie et al. 2018) and (259.2-)230.3, 226.9(-196.7) Ma (Quiroga et al. 2016) - note the topologies. Leslie et al. (2018) estimated crown group diversification to begin (223.6-)189.7(-159.5) Ma while the comparable ages in Sudianto et al. (2018) are 142-134 Ma and in Herting et al. (2020) they are 224-105 Ma.
Distinctive podocarp root nodules are known from very well-preserved fossils from ca 240 Ma in the Early Triassic of Antarctica (Schwendemann et al. 2011), while vegetative podocarp fossils with longitudinally-oriented para- and tetracytic stomata have been described from end-Permian deposits in Jordan perhaps 260-251.9 Ma (Blomenkemper et al. 2018).
1. Podocarpoideae Beilschmied
foliar stomata in longitudinal bands.
Age. Saxegothaea diverged from other Podocarpoideae (197.2-)164.7(-131.9) Ma (Leslie et al. 2018).
1A. Saxegothaeeae Gordon - Saxegothaea conspicua Lindley —— Synonymy: Saxegothaeaceae Doweld & Reveal
Plants monoecious; leaves petiolate, held vertically, ?isobifacial; pollination droplet 0; ovuliferous cone facing sideways [= lateral], two ovules/scale, nucellus protrudes from micropyle; pollen germinates on ovuliferous scale and tubes grow over the scale [extra-ovular caputure and germination]; several seeds/cone, + ovuliferous scales, ?not fleshy; n = 12.
1/1. South Chile and Argentina.
[Microcachrydeae [Pherosphaereae [Acmopyleae [Dacrydieae + Podocarpeae]]]]: ?
Age. The age of this node is (175.3-)143.0(-112.9) Ma (Leslie et al. 2018).
Distinctive fossil pollen (as Microcachrydites antarcticus, Podosporites parvus and other names) like that of Microcachrys is known from New Zealand ca 150 Ma (and since), and this pollen is widely distributed throughout the southern hemisphere including South Africa and along the Ninetyeast Ridge (references in Carpenter et al. 2011). Fossil foliage of Microcachrys, also distinctive, has quite recently been found in deposits 25.2-21.7 Ma from New Zealand (Carpenter et al. 2011).
1B. Microcachrydeae Y. Yang - Microcachrys tetragona J. D. Hooker —— Synonymy: Microcachrydaceae Doweld & Reveal
Subprostrate shrub; leaves opposite; plant dioecious; several seeds/cone, + ovuliferous scales; pollen trisaccate; unordered embryo cap cells; cleavage polyembryony.
1/1. Australia (Tasmania).
[Pherosphaereae [Acmopyleae [Dacrydieae + Podocarpeae]]: ?
Age. (158.1-)129.0(-99.8) is the estimated he age for this node (Leslie et al. 2018).
1C. Pherosphaereae Pilger - Pherosphaera Archer —— Synonymy: Microstrobaceae Doweld & Reveal, Pherosphaeraceae Nakai
Plant dioecious; pollen trisaccate; unordered embryo cap cells; ?seeds; cleavage polyembryony.
1/2. Australia: New South Wales and Tasmania.
Age. These two species may have diverged (45.3-)21.5(-4.1) Ma (Leslie et al. 2018).
[Acmopyleae [Dacrydieae + Podocarpeae]]: fleshy epimatium (0) and receptacle (0).
Age. This node is some (137.6-)112.1(-86.4) Ma (Leslie et al. 2018).
1D. Acmopyleae Y. Yang - Acmopyle Pilger —— Synonymy: Acmopylaceae Melikian & A. V. Bobrov
Leaves dimorphic, laterally flattaned, held in a single plane; plant dioecious; microsporophylls spirally arranged, triangular; female cones solitary, receptacle irregular, fleshy, verrucose; seeds solitary, nearly erect; n = 10.
1/2. New Caledonia and Fiji.
Age. Divergence here is thought to have happened (86.7-)51.0(-14.7) Ma (Leslie et al. 2018).
Acmopyle grayae has recently been described from deposits ca 52 Ma in southern Argentina; other Argentinian fossils previously placed in this genus have been moved to Dacrycarpus (Andruchow-Colombo et al. 2023a).
1E. Dacrydieae Gordon —— Synonymy: Bracteocarpaceae Melikian & A. V. Bobrov, Dacrycarpaceae Melikian & A. V. Bobrov, Falcatifoliaceae Melikian & A. V. Bobrov
Leaves (opposite), (laterally flattaned, held in a single plane); pollen (trisaccate - Dacrycarpus); n = 10.
3/43: Dacrydium (25). China (Hainan) and Myanmar to New Zealand, New Caledonia and Fiji. Photos Collection.
Age. Crown Dacrydieae are (99.1-)79.9(-62.8) Ma (Leslie et al. 2018).
Fossils of Dacrycarpus have been reported from the Jurassic of India (Florin 1940), and if correctly identified, this would push back ages in Podocarpaceae... More recent Patagonian fossils are younger, Early/Middle Eocene in age - ca 52.2 and 47.7 Ma are the ages of the two deposits involved - and they consist of attached cones and foliage, the former subtended by distinctive warty modified leaves (= a podocarpium), the whole clearly Dacrydium. Indeed, named D. puertae, Wilf (2012), who described the material, considered it to be indistinguishable from the extant D. imbricatua, from Southeast Asia and Malesia. Fossil pollen of Dacrycarpus is known from ca 73 Ma deposits in the Australian Otway basin, while pollen of Dacrydium is known from other Australian deposits ca 85 Ma.
1F. Podoarpeae Dumortier —— Synonymy: Nageiaceae D. Z. Fu
Leaves (opposite, held in a single plane) lamina (multiveined, branching dichotomous - Nageia); n = 10-13, 17-19.
4/125: Podocarpus (115). South Temperate, tropical montane, West Indies, New Caledonia, Fiji. Photos Collection.
Age. The age of this clade is (88.7-)67.5(-49.9) Ma (Leslie et al. 2018).
Retrophyllum, which still grows in southern South America, is known fossil from early end-Cretaceous Argentina 67-66 Ma (Wilf et al. 2017b; see also Wilf 2020). Fossil pollen of Podocarpus may be quite elderly (Dettmann & Jarzen 1990).
2. Phyllocladoideae W. Hochstetter
Leaves petiolate or not; plants dioecious (monoecious); exine alveolate/honeycomb; ovuliferous scales not aggregated into cones, ± reduced, fused with ovule and ± enveloping it [looking like an integument]; (ovule erect); pollination droplet spreads along surface of scale, scavenges pollen; ovuliferous scale fleshy [= epimatium].
Age. Crown Phyllocladoideae are (164.3-)125.3(-89.0) Ma (Leslie et al. 2018).
2A. Phyllocladeae Dumortier —— Synonymy: Halocarpaceae Melikian & A. V. Bobrov, Lepidothamnaceae Melikian & A. V. Bobrov, Parasitaxaceae Melikian & A. V. Bobrov, Phyllocladaceae Bessey
(Pollen not saccate - Phyllocladus); fleshy (epimatium 0, aril + - Phyllocladus); n = 9.
6/12: Phyllocladus (5), Borneo and the Philippines to New Guinea, Tasmania, New Caledonis, S. Chile, esp. New Zealand. Photos Collection, Phyllocladus trichomanoides, Phyllocladus megasporangia, microsporangia].
Age. Huncocladus, in deposits ca 52 Ma from Patagonia, Argentina, is thought to be close to Phyllocladus, the latter now a member of the Antipodean-Malesian flora (Andruchow-Colombo et al. 2019, see also 2023). Although Dörken et al. (2021) questioned the identity of this fossil, suggesting that it might be a cycad, Andruchow-Colombo et al. (2021) provided good evidence that it was correctly identified.
2B. Prumnopityeae Y. Yang —— Synonymy: Prumnopityaceae Melikian & A. V. Bobrov
Leaves spiral, appearing 2-ranked; plant dioecious; ovule 1/bract, erect; receptacle not fleshy; n = 19 (18).
3/10: Pectinopitys (6). Malesia (not the Malay Peninsula) to New Zealand, Fiji, New Caledonia, Costa Rica and W. South America.
Age. Prumnopityeae are (112.3-)80.0(-49.5) Ma (Leslie et al. 2018: as Prumnopitys).
Evolution: Divergence & Distribution. Andruchow-Colombo et al. (2023b) provide a critical evaluation of the fossil record of material associated with Podocarpaceae. Early fossils placed in the family include material from Jordan with paratetracytic stomata and leaves forming a spray by twisting of the petioles; the material, in coastal lowland deposits with a pronounced dry season and identified as "Podocarpaceae", has been dated as late Permian ca 253 Ma (Blomenkemper et al. 2018), however, Andruchow-Colombo et al. (2023b) question its identity. Fossil Podocarpaceae (as Rissikia) are known from the Middle Triassic of Antarctica ca 225 Ma, although the material has since been lost, but organ genera have been associataed with it (apparently the fossils had 2 ovules/scale, see also Saxegothaea: Townrow 1967; Eckert & Hall 2006; Axsmith et al. 1998; Biffin et al. 2011b: Suppl. 4; Rothwell et al. 2012). This material is more probably to be associated with Voltziales (Andruchow-Colombo et al. 2023b). However, there are Jurassic fossils like Harrisiocarpus cracovensis and Scarburgia blackii (from Poland and England respectively) that seem to be more reliably associated with Podocarpaceae (Andruchow-Colombo et al. 2023b).
Until the end of the Eocene Podocarpaceae were very common and diverse in southern Patagonia and they dominated Antarctic forests, but they were then replaced with Nothofagaceae - both are members of the Austral flora; for the biogeography, etc., of these podocarps see e.g. Mill (2003), Kooyman et al. (2014), Pujana and Ruiz (2017) and Carpenter and McLoughlin (2025). Indeed, Podocarpaceae, along with Araucariaceae, are the commonest gymnosperm fossils in Antarctica (de la Estrella et al. 2019b) while Nothofagus is the commonest angiosperm. Evidence from pollen and chemistry (diterpenoids) suggests that podocarps were growing in Antarctica ca 14 Ma or yet more recently (deposits from the Sirius Group, Transantarctic Mountains: Rees-Owen et al. 2018). These New World Palaeocene/Eocene podocarps from Argentina and elsewhere that now are restricted to the Old World include Acmopyle, Microcachrys and Dacrycarpus, as mentioned above (see also Kooyman et al. 2014; Merkhofer et al. 2015); Acmopyle in particular is noted for its current restriction to mesic habitats (Andruchow-Colombo 2023a; for early work on the genus, see Sahni 190). Such disjunct distributions, in part Gondwanan (they are probably attained by migration around Antarctica), are also discussed elsewhere. Interestingly, there are also well-substantiated reports of podocarps from the northern hemisphere in the Cretaceous and Early Caenozoic, e.g. Prumnopitys anglica, known from Eocene deposits in southeast England (Greenwood et al. 2013 and references). On the other hand, Pujana and Ruiz (2017) note that Podocarpoxylon fossils, quite common in the northen hemisphere, are unaccompanied by other remains that might be placed in Podocarpaceae and so are themselves unlikely to be Podocarpaceae, while this was not the case with fossils of that genus from the southern hemisphere. Dörken et al. (2021) evaluated the fossil record of Podocarpus.
L. Chen et al. (2021) suggested that the ancestral area of Podocarpaceae was Australia-New Zealand, and they then spread over the southern hemisphere with subsequent vicariance. The family is still quite common in the southern hemisphere and may dominate the vegetation in places, but as Australia has dried out during the Caenozoic, they have become less common there (e.g. Brodribb & Hill 1997, 2004; Biffin et al. 2011b). Diversification in clades whose members have imbricate leaves began in the Late Jurassic ca 150 Ma (Biffin et al. 2011b) or even earlier (Quiroga et al. 2016), and although diversification in clades whose members have flattened foliage is notably greater, it is younger, being dated to (94-)64(-38) Ma (Brodribb & Hill 2004; Biffin & Lowe 2011; Biffin et al. 2011b; Brodribb & Feild 2010), Chen et al. (2021) noting an increase of diversity of the [podocarp + dacrydioid] clade which they estimate has a crown-group age of around 120 Ma. Crown-group Podocarpus, with 107 species representing well over half the family, is (81.8-)65.2, 60(-52.2) Ma, i.e. late Cretaceous or early Palaeogene, although it could be up to 37 Ma older (Quiroga et al. 2016). Leslie et al. (2012) and Quiroga et al. (2016) offer more dates for splits within Podocarpaceae. Morley (2011) thought that Dacrydium might have moved into South East Asia via the Ninety East Ridge and India. For possible roles of New Caledonia and New Zealand in the persistence of Podocarpaceae, see Condamine et al. (2016: metapopulations on ephemeral islands?).
The [Dacrydieae + Podocarpeae] and Phyllocladoideae in particular have fleshy seed cones - this fleshiness is produced by structures that vary in morphology - receptaculum or epimatium, the latter sometimes fusing with the testa. The trees themselves have broad, flattened leaves, and all in all the plants can compete with angiosperms in the southern forests in which they grow (Khan et al. 2023).
There is evidence that the pollen cones of Podocarpaceae are pseudanthial in nature (Dörken 2024).
Ecology & Physiology. Podocarps are slow-growing, long-lived, light-demanding specialists that often grow in nutrient-poor soils, but are mostly poorly adapted to dessication stress (see e.g. Andruchow-Colombo et al. 2023a), and they can be dominants or emergents in southern forests (Brodribb 2011; Coomes & Bellingham 2011). Extant Podocarpaceae with broad leaves are shade tolerant and prefer warmer and higher rainfall conditions, and they also have fleshy diaspores, the often brightly-coloured seed having a fleshy epimatium (Farjon 2018). Foliage variation in Podocarpaceae is considerable. Biffin and Lowe (2011, see also Biffin et al. 2011b) suggest that podocarps with broad leaves or functionally equivalent structures like the phylloclades of Phyllocladus evolved about four to six times, probably slightly after the venation density of angiosperm leaves increased, i.e. (94-)64(-38) versus 109-60 Ma, interestingly, there have been reversals to imbricate leaves (see also L. Chen et al. 2021). In taxa like the spiral-leaved Afrocarpus the leaves orient themselves to form a spray of foliage (Andruchow-Colombo 2023a for other examples), all leaves having their upper surfaces uppermost in the spray, but in the amphistomatous Retrophyllum all leaves twist in the same direction so the upper surface may face the lower side of the spray (Wilf et al. 2017b). Broad-leaved podocarps are now largely meso-megathermal shade-tolerant plants (high rates of transitions from microthermal habitats) while the imbricate-leaved taxa are mostly microthermal (high rates of transitions from meso-megathermal: Biffin & Lowe 2011; Biffin et al. 2011b; Brodribb & Feild 2010). Accessory transfusion tissue, perpendicular to the midvein, is common in broad-leaved podocarps (it is a family apomorphy above, albeit a bit tentative) and facilitates the movement of water in such leaves (Andruchow-Colombo et al. 2023a). Fossils with apparent affinities to podocarps and with broad leaves are known from the Triassic and Jurassic (Biffin et al. 2011b).
Podocarp leaves are recalcitrant, decomposing only slowly (although there are few studies on this); there is a high concentration of condensed tannins (Ushio et al. 2017) and carbon/lignin build up in the soil, nutrients are sequestered and soil fertility is further reduced (Wardle et al. 2008; Dickie & Holdaway 2011); they have been described as ecosystem engineers because of this combination of features (Coomes & Bellingham 2011). Soils beneath the tree may be low in both N and P, but Dacrydium gracilis, at least, effectively removes the little inorganic N that is there, while saprophytic microbes may contribute to the high acid phosphatase activity in the soil which relieves the P limitation (Ushio et al. 2017).
For root nodules, see above.
The New Caledonian Parasitaxus usta (Parasitaxoideae) is hemiparasitic on the roots of Falcatifolium taxoides (Podocarpoideae), where it taps the xylem and from which it obtains water and nutrients (the stomata of Parasitaxus are insensitive to light). The plant is a mycoheterotroph, obtaining carbon from an ?ectomycorrhizal fungus that is also associated with its host and whose hyphae grow through the vascular systems of both host and parasite (Woltz et al. 1994; Feild & Brodribb 2005; Merckx et al. 2013a; Imhof et al. 2013a). The stem-group age of Parasitaxus is ca 154 Ma, far older than that of its host (L. Chen et al. 2021). Parasitism is at least partly indirect here. Its seed is surrounded by a globose, hard, glaucous, white epimatium,
Pollination Biology & Seed Dispersal. There is a correlation between the absence of pollen wings and the shedding of the pollen exine when the microgametophyte germinates. In Phyllocladus, which has erect ovules, the pollination droplet is actively resorbed (see Tomlinson et al. 1991, esp. 1997: useful comparisons; Rydin & Friis 2005). For pollen tube growth in Saxegothaea, see J. Doyle and O'Leary (1935a); if that genus is sister to the rest of the family, one may have to rethink aspects of the evolution of pollination mechanisms in Podocarpaceae (c.f. Leslie et al. 2015). See also Little et al. (2014) for pollination.
For details of the complex seed morphology of Podocaerpaceae, seed dispersal, etc., and their evolution, see Contreras et al. (2016: fig. 2), L. Chen et al. (2021: fig. 5), Nigris et al. (2021) and Khan et al. (2022, 2023). Diaspores in the family may have been ancestrally dry (but see topology of the trees; Khan et al. 2023), but in bird- and mammal-dispersed taxa the receptacle and/or epimatium/ovuliferous scale have become fleshy and variously developed and they more or less surround the seed.
Vegetative Variation. Phyllocladus has phylloclades, i.e. flattened, photosynthetic stems; these bear highly reduced, scale-like leaves the vascular supply to which which may lack leaf gaps, and it is in the axils of these leaves that the reproductive structures are found. However, the seedling has more conventional needle-like leaves. The foliar units of podocarps with flattened foliage units, whether leaves or phylloclades, have transfusion tissue or there are several veins, unlike the single vein and absence of transfusion tissue in the leaves of other podocarps (Tomlinson et al. 1989b; Biffin et al. 2011b; Dörken et al. 2021).
Genes & Genomes. There are early suggestions that n = 20 ((Hair 1963). Quinn et al. (2002) noted that dysploid chromosome evolution was quite common in the group.
Sudianto et al. (2018) discuss the plastome of Podocarpaceae, which varies in size from 121.1-151.5 kb, that of Lagarostrobus being the largest, and that genus has notably high intergenic spacer content and abundant repeats. Although Parasitaxus has chlorophyll a and b, it has lost its genes for photosynthesis, furthermore, its light-independent chorophyll biosynthesis genes chlB, chlL and chlN have become pseudogenized; its plastome has regained an IR of 9,256 bp long (X.-J. Qu et al. 2019). Isomeric plastomes are repoted from Podocarpaceae (see C. Lee et al. 2020).
Chemistry, Morphology, etc.. For secondary metabolites in Podocarpus s.l., see Abdillahi et al. (2010); taxol has been found in Afrocarpus gracilior (fungi involved here, too?). Syringyl lignins have been reported quite widely from Podocarpaceae, although mostly from subepideraml fibres and sclereids (e.g. Gibbs 1958). There is a single record of whethher or not root cortical cell walls have phi thickenings (Gerrath et al. 2002). For cuticular features and how they may be affected by the environment, see Clugston et al. (2017). Accessory transfusion tissue extends to the lamina margin in Podocarpus macrophyllus and a number of other species of the genus (Gifford & Foster 1989); Knopf et al. (2012) provide many details of foliar anatomy for the whole group.
Although the single ovules of most Podocarpaceae do seem very different from the multiovulate cones of most other Pinales, Lower Cretaceous podocarps with more conventional bract-scale complexes, as in the extant Saxegothaea, have been described (X. Wang et al. 2008). The morphological nature of the epimatium has occasioned some controversy. Chamberlain (1935) interpreted it as possibly being equivalent to the ovuliferous scale, probably a reasonable interpretation (see also Tomlinson & Takaso 2002; Englund et al. 2011: similarity confirmed by gene expression data), and functionally, perhaps, it can be considered equivalent to the second integument of an angiosperm ovule - hence the "anatropy" of the ovules here (Endress 2011b). The epimatium may be free from or adnate to the integument (see also Contreras et al. 2016 for seed morphology). Phyllocladus is sometimes described as having an aril, although this is more probably a somewhat reduced and retarded epimatium (de Laubenfels 1988); Tomlinson and Takaso (1989) found evidence that the structure was paired, c.f. modified leaves, also Taxaceae? Khan et al. (2022: see also Leslie 2023) clarified the variation and evolution of the various fleshy structures (aril, epimatium, bracts, receptaculum) in the prumnopityoid clade (= Phyllocladoideae).
Although the pollen of Phyllocladus has often been described as having a wing (e.g. H. Singh 1978), a wing seems to be absent. For details of embryogeny, variable and complex (and not integrated above), see Buchholz (1941) and Roy Chowdhury (1963), and for nucleus number in the E-tier cells, see also Quinn (1986). Cleavage and true polyembryony are common in Podocarpaceae, indeed, embryos seem able to develop from almost any cell of the early embryo (Buchholz 1941; for polyembryony, see also J. Doyle and Brennan (1972: integrate this character better).
For general information, see Turner and Cernusak (2011: Smithsonian Contrib. Bot. 95. 2011) and the Gymnosperm Database, also Page (1990: Phyllocladaceae, Podocarpaceae) and for Podocarpus, see Mill (2014: literature summary); for cuticle morphology, see Mills and Schilling (2009), for wood anatomy, see Woltz et al. (2009 and references), for shoot growth, see Stoffberg (1991a), for leaf traces, see Axsmith et al. (1998), for ovules, etc., see Stoffberg (1991b) and for Phyllocladus see Quinn (1986: embryogeny) and Tomlinson et al. (1989a: cone, etc.).
Phylogeny. Relationships of three genera in particular, Phyllocladus, Parasitaxus and Saxegothaea, have occasioned a considerable amount of comment (for early literature, see Sahni 1920). 1. Phyllocladus. Kelch (1998) provided an early comparison of morphology and molecules. RbcL analyses (Conran et al. 2000; Wagstaff 2004b) tended to place Phyllocladus within Podocarpaceae, other analyses, whether (Quinn et al. 2002) or not (Sinclair et al. 2002) including rbcL sequences, placed the two as sister groups. Indeed, in Sinclair et al. (2002) relationships were [Phyllocladus [Lepidothamnus [[Prumnopitys [Lagarostrobus, Parasitaxus, etc.]] [Saxegothaea [Falcatifolium, etc.]]]]]. Inclusion of Phyllocladus within rather than sister to all other Podocarpaceae is likely, as in Peery et al. (2008: nuclear XDH gene), and it was included in the small prumnopityoid clade in the combined analysis of Knopf et al. (2012: support in/for this clade not strong in single gene analyses; see also Biffin et al. 2011a, b; Quiroga et al. 2016). Leslie et al. (2018), but not Y. Yang et al. (2022), recovered a clade [Phyllocladus + Lepidothamnus that was sister to other Phyllocladoideae. 2. Parasitaxus. The closest relatives of Parasitaxus are Lagarostrobus and Manoao, both monotypic and from Tasmania and New Zealand respectively - [Parasitaxus [Lagarostrobus + Manoao]] (Sinclair et al. 2002; Rai et al. 2009; Lam et al. 2009; Sudianto et al. 2018; Leslie et al. 2018; Yang et al. 2022; c.f. Quiroga et al. 2016: Lagarostrobus in a different part of the family). Halocarpus is often close, although further details of relationships vary. Y. Lu et al. (2014) even suggested that Parasitaxus and Phyllocladus were sister taxa. 3. Saxegothaea. This has some support as being sister to all the rest of the family (Knopf et al. 2012, but c.f. Biffin et al. 2011a, b; Y. Lu et al. 2014; Quiroga et al. 2016: sister to [Microcachrys [Pherosphaera [Acmopyle + ...]]]; Leslie et al. 2012, 2018: sister to the Tropical Clade). Both Leslie et al. 2018) and Yang et al. (2022) placed Saxegothaea sister to the rest of Podocarpoideae, and with strong support. Its final resting place will have considerable implications for character evolution in Podocarpaceae; Mabberley (2007) noted that Saxegothaea has some features reminiscent of Araucariaceae (see also Sahni 1920). Other groupings of genera are becoming evident (Kelch et al. 2010), including the dacrydioid and podocarp clades (Knopf et al. 2012) - as tribes, see Yang et al. (2022); for a morphological phylogeny of the family that agrees quite well with the phylogeny above, see Andruchow-Colombo et al. (2023a). Relationships in the Podocarpus-Sundacarpus-Parasitaxus-Phyllocadus area recovered by Stull et al. (2021) were poorly supported.
Sudianto et al. (2018) analysed plastomes of Podocarpaceae and used variation in 81 genes to disentangle relationships, finding i.a. that Saxegothaea was sister to Podocarpoideae, not to the whole family. L. Chen et al. (2021) looked at both nucleome (993 genes) and plastome (54 genes) sequences from 44 species (all genera). The relationships that they obtained were for the most part strongly supported, and can be summarized as follows [[prumnopityoids - 7 genera] [Saxegothaea [Microcachrys [Pherosphera
[Acmopyle [[dacrydioids - 3 genera] + [podocarpoids - 4 genera]]]]]]; both Phyllocladus and Parasitaxus were embedded in the prumnopityoids, but the position of Halocarpus was rather unsettled (Chen et al. 2021).For relationships within Podocarpus in particular, see Biffin et al. (2011, 2012), Quiroga et al. (2016) and Leslie et al. (2018), and for relationships in Dacrydium, D. cupressinum sister to the rest. see Leslie et al (2018).
Classification. The classification above is taken from that in L. Chen et al. (2022); the four monotypic tribes in Podocarpoideae are necessitated by the pectinate nature of the tree there. For generic limits in Podocarpaceae, see also Page (2019).
Previous Relationships. Phyllocladus was long considered to be very distinctive, so distinctive that it was sometimes separated from all other conifers (e.g. Keng 1974, 1979).
[Sciadopityaceae [Taxaceae + Cupressaceae]]: roots with endomycorrhizae; root cortical cell walls with phi [φ] thickenings; secretory cells in the centre of the root [?or next node up]; two-year reproductive cycle/three-year, fertilization in 2nd/3rd year; pollen without sacci, not buoyant, exine shed on germination [microgametophyte naked]; ovuliferous cone horizontal to pendant, with 10≥ basal sterile scales; bract scale becoming narrowed at the base; ovuliferous scale adnate to base of bract scale, ovules erect, becoming inverted; prothallial cells 0; seed winged, wing developing from the integument, narrow; scales reflex at maturity.
Age. Leslie et al. (2012) estimated an age for this node of over 250 Ma, some time in the late Permian, and Leslie et al. (2018) put the age at (276.6-)248.3(-222.1) Ma; ca 230 Ma is the estimate in Ran et al. (2018a) and 337-157 Ma that of Herting et al. (2020).
Evolution: Genes & Genomes. The occurence of chloroplast genome isomers is scattered in this clade, but they are particularly common in Cupressoideae (Hsu et al. 2016; Qu et al. 2017), and are also common in angiosperms and at least some ferns (W. Wang & Lanfear 2019).
Chemistry, Morphology, etc.. The pollen grains expand and rupture when placed in water (Tomlinson 1994), and the intine-clad pollen, although sometimes much larger than the pollen grain itself, may deform more easily and so be transferred along the narrow micropylar canal (Takaso & Owens 2008). Whether or not all taxa have male gametes each surrounded by cell walls needs to be confirmed (see H. Singh 1978).
SCIADOPITYACEAE Luersson - Sciadopitys verticillata (Thunberg) Siebold & Zuccarini - Back to Cupressales
Tracheid pits with margo only, wood parenchyma 0, walls of tranverse and tangential ray cells not pitted; short shoots ultimately abscise; 8-12 subsidiary cells/stoma; leaves on long shoots reduced to scales; short shoots +, subtended by a scale leaf, with a pair of connate needles, apically bifid (not), vascular bundles surrounded by sheaths, petiole 0; pollen cones in clusters/pseudowhorls, microsporophyll with flattened apical expansion, (1-)2 microsporangia/microsporophyll; pollen inaperturate, surface verrucate, microtuberculate/microechinate, verrucae confluent by sporopollenin deposition, ± perforate; male gametophyte: sterile cell?, male gametes unequal; ovules (1-)7-9(-12)/bract scale, margin of ovuliferous scales fringed, ± connate; pollen chamber?; proembryo with 5/6 nucmlear divisions, cleavage polyembryony extreme; seeds (1-)7-9(-12)/scale; n = 10, nuclear genome [1 C] ca 20 pg, chloroplast accD gene to nucleus.
1/1: [list]. C. and S. Japan. Map: from Florin (1963). [Sciadopitys Photos - Collection]
Evolution: Divergence & Distribution. Fossils of Sciadopitys are known from the Upper Cretaceous and afterwards, but apart from material from Japan and the Upper Pliocene of Germany, the identities of many are questionable (Stockey et al. 2005). Pollen, Sciadopityspollenites and Cerebropollenites, can be linked with that of Sciadopitys, but the pollen in Tsuga is rather different; Cerebropollenites is known from as far back as the late Triassic (Hofmann et al. 2021a).
Ecology & Physiology. Resin from Sciadopitys may have contributed to Baltic amber 47-34 Ma (Sadowski et al. 2016a), indeed, Sciadopitys may be the main source of resin for this amber - all ca 105 tons of it (A. P. Wolfe et al. 2009), but Pinaceae, Araucariaceae and perhaps Cupressaceae may also have contributed (Seyfullah et al. 2018).
Vegetative Variation. There has been much debate over whether the photosynthesising structures of Sciadopitys are some kind of phylloclade - perhaps formed by the connation of two leaves - or cladode, a modified stem structures. The two vascular bundles, each with its own endodermis, tend to have have abaxial xylem and adaxial phloem, perhaps a rather odd arrangement for a leaf-derived structure. Sporne (1965) noted that on occasion branches develop from these leafy structures, so perhaps favoring the cladode hypothesis (see also Farjon 2005c). However, Dörken and Stützel (2011) examined probably only the second known case of intermediate structures and considered the "needles" to be two congenitally connate needle leaves, the orientation of the vascular tissue reflecting the relation of the leaves to the axis that bore them (see also Dörken & Stützel 2012a). The "needle" is thus comparable with the fascicles of separate needles in Pinaceae.
Genes & Genomes. For the distinctive chloroplast genome of Sciadopitys with two inverted repeats and the formation of four chimaeric gene clusters, see J. Li et al. (2016) and Hsu et al. (2016). The chloroplast accD gene has moved to the nucleus (Sudianto & Chaw 2019), perhaps soon after Sciadopitys split from the other Cupressales (J. Li et al. 2016). Isomeric plastomes are reported from Sciadopitys (C. Lee et al. 2020).
Chemistry, Morphology, etc.. There are apparently no resin canals in the wood (Pierce 1935); resin exudes from the pith and cortex in the cones and appears to be exuding from the cortex of twigs/small branches (A. P. Wolfe et al. 2009: Fig. 4j, k).
For a monograph, see Farjon (2005c), and for general information, see also Gymnosperm Database, for pollen, see Page (1990) and Uehara and Saichi (2011), for ovule and cone, see Takaso and Tomlinson (1991) and for embryo development, see J. Doyle (1963) and Roy Chowdhury (1963).
[Taxaceae + Cupressaceae]: subsidiary cells 4-7/stoma; lamina vascular bundles ?surrounded by sheath; microsporangia hypodermal in origin; ovules not borne on predeveloped axillary structures; chloroplast trnQ IR +.
Age. Leslie et al. (2012) estimate that these two families diverged 217-197 Ma, Y. Lu et al. (2014) estimated an age of ca 211.5 Ma, Leslie et al. (2018) put this age at (220.7-)205.2(-197.1), Mao et al. (2012) offer an age of (293-)245, 242(-194) Ma, while estimates in Yang et al. (2012) are 217-197 Ma (or perhaps 237 My), in Won and Renner (2006) they are (265-)227(-189) Ma, while in Magallón et al. (2013) they are ca 175.4 Ma; ca 208 Ma is the figure in Ran et al. (2018a), (199.0-)197.0(-195.1) Ma in Ji et al. (2020), (276.2-)243.7(-216.8) Ma in X.-Q. Liu et al. (2022) and ca 191.6 Ma in Fu et al. (2023).
Evolution: Ecology & Physiology. The evolution of cavitation-resistant xylem and stomata that stay open during periods of drought (Brodribb et al. 2014) can perhaps be placed at this node - Cunninghamia, Sequoiadendron and Glyptostrobus are examples, although not all species show this combination of characters. See also Callitris for an extreme example of this.
Gene expression studies suggest that there is little in common between the scaly structures of those Cupressaceae cones studied and the arils of Taxus with the ovuliferous scales of Pinaceae... (Englund et al. 2011; Groth et al. 2011).
Genes & Genomes. There appear to have been three genome duplications at this node (Z. Li & Barker 2019/2020).
Chemistry, Morphology, etc.. Cunninghamia and Taxus had by far the thickest roots (roots developing after root pruning) of the fourteen species of seed plants examined (B. Liu et al. 2015). Burrows (2009) noted that axillary buds occur in several members of this clade, but they were superficial and so did not form shoots in older branches - c.f. Araucariaceae.
TAXACEAE Berchtold & J. Presl - Back to Cupressales
Bands of fibres in phloem crystalliferous; leaves shortly petiolate; plant dioecious (monoecious); male gametophyte: pollen grains inaperturate, shed at 1-cell stage, 4-nucleate - tube cell/sterile cell/gametes, gametes unequal in size; ovuliferous cone pendulous, scales opposite; (ovules erect), pollen chamber +; disseminule fleshy ["arillate"], testa vascularized, with sarco- and sclerotesta; x = ?12, nuclear genome [1 C] 11.5-30 pg.
6/30: [list]. Northern Hemisphere, scattered, also New Caledonia.
Age. Estimates of the age of this node vary - e.g. (247.4-)210.5(-173.6) Ma (J. Liu et al. 2013), (231-)187(-144) Ma (Won & Renner 2006), (201.8-)170.1(-131.1) Ma (Leslie et al. 2018), ca 144.4 Ma (Y. Lu et al. 2014), ca 134 Ma (Ran et al. 2018a), 197-91 Ma (Herting et al. 2020), (165.5-)163.5(161.6) Ma (Ji et al. 2020) or (183.2-)127.0(71.6) Ma (X.-Q. Liu et al. 2022).
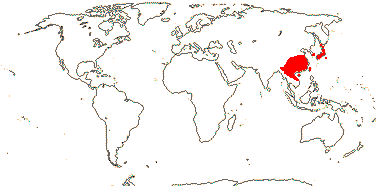
1. Cephalotaxeae Wettstein - Cephalotaxus Endlicher —— Synonymy: Cephalotaxaceae F. W. Neger
Cephalotaxine- and homoerythrine-type al-kaloids +; tracheids with margo-torus pits; leaves ±opposite, 2-ranked by twisting, 1 resin canal ventral to midvein; male cones in globose groups, 2-3 microsporangia/microsporophyll, terminal microsporangiophores perisporangiate, with several sporangia; female inflorescence spicate, 3–8 pairs of decussate bracts, ovuliferous scale much reduced, ovules 2/bract scale, ridge between them, vascular bundles 2/ovule, inverted; seed takes 2 years to develop [pollen tubes dormant]; female gametophyte with 1024-4096 free nuclei; cap cells of embryo degenerate; n = 12. [Ceph. embryos fromm prosuspensor cells; prosuspensor and secondary suspensor; male gam. tube nucleus, stalk + body cells, latter 2 gametes; micropyle closed by active growth of innermost integumentary cells.]
1/6. E. Himalayas to Japan. Map: from Florin (1963). [Cephalotaxus koreana Photos - Collection, C. fortunei, Collection.]
Age. Crown Cephalotaxeae are dated at (38.3-)22.9(-10.4) Ma (Leslie et al. 2018) and (39.3-)20.9(-8.5) Ma (Ji et al. 2020).
2. Taxeae Duby —— Synonymy: Amentotaxaceae Kudô & Yamamoto, Austrotaxaceae Nakai, Torreyaceae Nakai
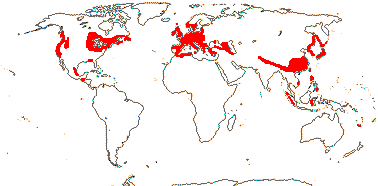
Taxine and pseudoephedrine-type alkaloids; tracheid pits with margo alone; sclereids + [Taxus]; leaves lacking resin canals; male cones with perisporangiate microsporangiophores, or with 2 hyposporangiate sprangia, (pherophylls 0 [Tax., Torr.]; bract and ovuliferous scale 0, ovule solitary, on shoot in axil of vegetative leaf, vascular bundles normal orientation, aril from fusion of 2 bracts, or 3, arrangement cyme-like, (rudimentary pollen chamber +); female gametophyte with ca 256 free nuclei; embryo short/minute (cotyledons 3); n = (7, 8, 11) 12.
5/32: Taxus ((10-)15(-24)). Scattered in the Northern Hemisphere, esp. South East Asia, also New Caledonia. Map: from Florin (1963) and de Laubenfels (1988). Photos: Collection.
Age. The age of Taxeae is thought to be some 138 Ma (Magallón et al. 2013) or (160.2-)144.5(-126.1) Ma (Ji et al. 2020).
Evolution: Divergence & Distribution. Möller et al. (2020 and refs) give ages for various clades here.
Möller et al. (2020) discussed relationships in Taxus; migration and hybridization have played major roles in shaping the evolution and distribution of the genus.
Taxus and its immediate relatives have female cones with one or a few ovules and the seed is surrounded by an aril-type structure whose nature has occasioned much discussion. In a reinterpretation of the female reproductive structures Stützel and Röwekamp (1999a) suggested that Taxus in particular can be linked with Torreya and then to other conifers; the single ovule of Taxus is in fact axillary. The sarcotesta of Cephalotaxus has been tentatively equated with the aril of Taxus (Mundry 2000), although the two would not seem to be homologous. On the other hand, Dörken et al. (2018a), emphasizing terata in Pseudotaxus chienii, suggested that the aril there might represent a pair of modified fused leaves, although there is no vascularization (c.f. Phyllocladus); they start developing only when the ovule is already large. In other Taxaceae this aril-type structure is adnate to the ovule. See also Nigris et al. (2021) for more on fleshiness and the seeds of Taxaceae, Leslie et al. (2018) for ovule evolution and Y. Yang et al. (2023) for a detailed discussion of all aspects of variation in the family.
And then there is the issue of nature of the male cones. It is suggested that the pollen cones of Taxus and Torreya are made up of several pseudanthia, while the male cones of Cephalotaxua are more obviously compound (see e.g. Mundry & Mundry 2001; especially Dörken et al. 2011; Dörken & Nimsch 2023; Dörken & Stützel 2024).
Pollination Biology & Seed Dispersal. Fleshy disseminules occur throughout Taxaceae (see Leslie et al. 2017).
Plant-Bacterial/Fungal Associations. Taxol and related compounds are synthesized by Taxus and also by several fungi that either grow in the soil around the plant or are endophytes (Cassady et al. 2004 and references); the fungi may have acquired the ability to synthesize taxol from the plant (Strobel et al. 1996). Pestalotiopsis guepinii, which can synthesize taxol, is also endophytic in Wollemia (Araucariaceae), etc. (Strobel et al. 1997).
Genes & Genomes. Torreya grandis has a notably larger nuclear genome that Taxus baccata - 18.1 vs 10.2 Gb (Y.-E. Lin et al. 2025).
Heteroplasmy is reported from Taxaceae, genes have been lost from the plastomes of Taxus and Pseudotaxus, and there are isomeric plastomes; this and other plastome variation makes species easy to identify (C.-N. Fu et al. 2019b).
Chemistry, Morphology, etc.. (S)norcolaurine synthase activity is high in both Cephalotaxus and other Taxaceae; this might suggest that benzyisoquinoline alkaoids may be found here (Liscombe et al. 2005). Cephalotaxus contains some very distinctive alkaloids (Parry et al. 1980).
Variation in cuticle/epidermis micromorphology is quite extensive (Elpe et al. 2017), as is that of leaf anatomy (Ghimire et al. 2014a), but they do not suggest broad relationships. The leaves of regenerating shoots of Cephalotaxus are initially spirally arranged (H. Singh 1961).
Taxaceae lack sacci on their pollen (Anderson & Owens 2006). The scales subtending the ovules of Austrotaxus are spiral. J. Doyle (1963) discussed the quite extensive variation in early embryo development in Taxaceae in some detail.
For a general account, see Page (1990: Cephalotaxaceae, Taxaceae), Cope (1998) and Y. Yang et al. (2023: Cephalotaxus), for the morphology of Taxus and relatives, see Hart and Price (1990), for male reproductive structures, see Wang et al. (2008) and Dörken and Nimsch (2016), for male gametes, see Chamberlain (1935) and H. Singh (1978), for the megasporangiate cone, see André (1956) and Liang and Wang (1989), and for embryology in general, see Roy Chowdhury (1963) and Chen and Wang (1990: male gametes somewhat to very unequal in size).
Phylogeny. Page (1990) included Amentotaxus in Cephalotaxaceae, although he noted that affinities between the two were "somewhat enigmatic"; indeed, a family so delimited appears para- or polyphyletic with relation to Taxaceae s. str., c.f. e.g. Price (2003), Hao et al. (2008), Leslie et al. (2018). Quinn et al. (2002) in a broad survey of Pinales found that Cephalotaxus, Torreya and relatives, and Taxus and relatives formed a tritomy in their unweighted rbcL and matK analyses; only when weighted were Cephalotaxaceae and Taxaceae separate. Price (2006) looked at variation in the same two genes and found weak support for Cephalotaxus as sister to [Amentotaxus + Torreya]; sampling overall was poor, but good for Taxaceae s.l., and support for the monophyly of Taxaceae s.l. was strong. These relationships were found by Wang et al. (2003) in analyses of trnL/F singly and when combined with rbcL data, but not in an analysis of rbcL alone, when Cephalotaxus alone was sister to all other Taxaceae; for the latter relationship, see also Rai et al. (2009), Leslie et al. (2018), Ji et al. (2021: plastome analyses), etc., and the latter group found [Torreya + Amentotaxus] to be sister to other Taxeae, while Leslie et al. (2018) found Austrotaxus to be in that position. Analyzing RNAseq data, Majeed et al. (2018) found that a clade [A [T + C]] was srongly supported as being sister to the rest of the family; see also Y. Lu et al. (2014: [C [T + A]]), although very different topologies were recovered in some analyses, while Y. Yang et al. (2022) found [T + A} to be in that position. Stull et al. (2021) found little support for the monophyly of Taxeae. For phylogenies, see also Cheng et al. (2000) and Ghimire and Heo (2014a: morphology only).
Classification. Cephalotaxaceae and Taxaceae have sometimes been separated, as by H. Singh (1961); he was unclear what the relationships of Cephalotaxus were, so he thought familial rank for it was appropriate, and this idea has also been supported by Hao et al. (2008), Majeed et al. (2018) and Y. Yang et al. (2023). However, the work of Rai et al. (2008a) is consistent with a broad circumscription of Taxaceae, as is that of Leslie et al. (2012) - of course, this is basically a ranking problem, so is largely trivial, and discussions like those of Yang et al. (2023: see esp. Fig. 5B) do not suggest changes in our ideas of the circumscriptions of the two groups above. Since relationships in the rest of the family have not settled down, I recognised an expanded Taxeae above - but anyhow, there are only five genera total in this area.
Previous Relationships. Taxus has sometimes been considered quite distinct from all other conifers, and, as Taxopsida, supposedly separate from Coniferopsida since perhaps the late Palaeozoic (Florin 1958, also Florin 1949, 1954; C. N. Miller 1999, c.f. 1977).
CUPRESSACEAE Bartling - Back to Cupressales
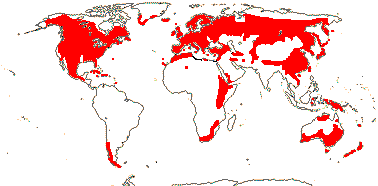
(Root stele pentarch); wood rays 10> cells tall; (stem apex with tunica/corpus construction); resin canals inducible in xylem or phloem [in separate clades]; cladoptosis + [?level]; foliar resin canal one or more, abaxial to vascular bundle; leaves ± amphistomatous, stomata arranged (±) longitudinally; leaves spiral, ± linear-acicular; pollen cones in clusters/pseudowhorls; (1-)2-10(-14) microsporangia/microsporophyll; pollen shed at 2-cell stage, with orbicules [?level]; male gametophyte: 3-nucleate [tube cell/gametes], gametes with cell walls; bract and seed scales ± evident, imbricate, ovules develop on the base of the subtending scale [= subaxillary]; seed dispersed with part of its supporting structure, with lateral wing; early embryo with 2-3 free-nuclear divisions; n = x = 11; chloroplast large inverted repeat 0, smaller IR duplicating the trnQ gene; mitochondrial transmission paternal.
32/143: [list - 7 subfamilies below]. Esp. Northern Hemisphere, more scattered in south temperate regions. Photos - Collection.
Age. Mao et al. (2012) offer an age of (259-)219, 211(-168) Ma for crown Cupressaceae, while estimates in Yang et al. (2012) are (231-)229, 197(-186) Ma and in Y. Lu et al. (2014) ca 197.1 Ma; ca 171 Ma is suggested by Ran et al. (2018a), (192.2-)170.4(-146.4) Ma by Leslie et al. (2018), 185-105 Ma by Herting et al. (2020) and (262-)229.5(-196) Ma in X.-Q. Liu et al. (2022).
Evolution: Plant-Animal Interactions.Megastigmus (Hymenoptera-Torymidae) is a particularly notable pest of conifers, and in species of Cupressaceae it lays its eggs only in fertilized seeds (c.f. Pinaceae: Roualt et al. 2004).
Jurassic fossils placed in crown-group Cupressaceae in morphological analyses, specifically in a clade along with Cunninghamia and Taiwania, in turn part of a pentatomy that made up the family as a whole (Escapa et al. 2008), are known from both Yorkshire, England - Elatides, Bajocian, ca 169 Ma (Harris 1943), and Chubut, Argentina - Austrohamia, early Jurassic 187-183 Ma (and also known from Inner Mongolia!!) (Escapa et al. 2008; Buczkowski et al. 2015; Bodnar & Escapa 2016; Contreras et al. 2019). There are cunninghamioid fossils from the Jurassic suggesting that this clade may be as old as the Triassic.
1. Cunninghamioideae Silba - Cunninghamia Richard —— Synonymy: Cunninghamiaceae Siebold & Zuccarini
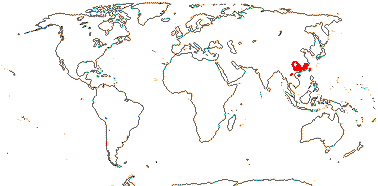
Tracheid pits with margo alone; eaves 2.5-6 cm long, twisted at the base, (margin denticulate), (hypostomatous), narrowed at base, petiole 0; pollen cones 16-many, growth of shoot continuing later [= proliferating], 3(-6) microsporangia/microsporophyll; ovuliferous cone initially pendulous, bract scales lacking adaxial stomata, ovules borne on predeveloped axillary structure, ovuliferous scale soon degenerating [small, 1/3> length of cone scale, free, margin initially 3-lobed], ovules 2-3(-6)/scale; seeds ca 2/bract scale; nuclear genome [1 C] ca 12.5 pg.
1/2. Laos, Vietnam, Southern China, Taiwan. Map: from Farjon and Filer (2013).
Age. The age of crown-group Cunninghamia is thought to be (4.4-)1.4(-0) Ma (Leslie et al. 2018) or (19.5-)10.8(-4.0) Ma (X.-Q. Liu et al. 2022).
Cunninghamia (as C. taylorii) is known from the Late Cretaceous in Alberta, Canada, in deposits perhaps ca 72.5 Ma (Serbet et al. 2013).
[Taiwanioideae [[Athrotaxidoideae + Sequoioideae] [Taxodioideae [Actinostroboideae + Cupressoideae]]]]: extensions of torus in tracheidal pit; leaves usu. 3> cm long; pollen cone shoot not proliferating.
Age. The age of this clade is estimated to be (179.9-)156.8(-132.5) Ma (Leslie et al. 2018) or (241.6-)210.9(-179.5) Ma (X.-Q. Liu et al. 2022).
2. Taiwanioideae L. C. Li - Taiwania Hayata —— Synonymy: Taiwaniaceae Hayata
Tracheid pits with margo alone; leaves shortly linear, not narrowed at base, petiole 0; pollen cones 2-7, axillary; bract scales with adaxial stomata, not narrowed at the base; ovuliferous cone initially erect, ovuliferous scale invisible; ovules (1-)2/bract scale, subaxillary; seeds ca 2/bract scale; nuclear genome [1C] ca 11.5 pg.
1/2. Northern Myanmar and Vietnam, S.W. China, Taiwan.
[[Athrotaxidoideae + Sequoioideae] [Taxodioideae [Actinostroboideae + Cupressoideae]]]: stomata usu. with Florin Rings; pollen surface microverrucate/papillate.
Age. This clade is some (167.7-)145.8(-121.9) Ma (Leslie et al. 2018: note topology), a mere 66.4 Ma (Magallón et al. 2013), ca 183.2 Ma (J. Li et al. 2021) or (220.7-)192.2(-162.5) Ma (X.-Q. Liu et al. 2022).
[Athrotaxidoideae + Sequoioideae]: bract-scale complex peltate.
Age. [Athrotaxidoideae + Sequoioideae] are estimated to be (212.7-)182.3(-151.6) Ma (X.-Q. Liu et al. 2022).
Pollen cones of stem-group Athrotaxis, A. ungeri, are reported from early Albian deposits perhaps ca 100 Ma in Patagonian Argentina; it was once quite widespread in the Southern Hemisphere (del Fueyo et al. 2008).
3. Athrotaxidoideae L. C. Li - Athrotaxis D. Don —— Synonymy: Athrotaxidaceae Doweld
Tracheid pits with margo alone; leaves scale-like to linear, not narrowed at base, petiole 0; pollen cone 1, terminal; ovuliferous cone initially ?, ovules borne on predeveloped axillary structure, ovuliferous scale soon degenerating, ovules 1-9/bract scale, adaxial thickening developing on adaxial side of bract scale above ovules after pollination [?= ovuliferous scale]; nuclear genome [1 C] ca 10 pg.
1/2. Tasmania.
Age. The species of Athrotaxis diverged (16.6-)6.8(-0.8) Ma (Leslie et al. 2018).
4. Sequoioideae Quinn —— Synonymy: Metasequoiaceae Hu & W. C. Cheng, Sequoiaceae Luersson
(Plant deciduous); xylem with inducible axial resin canals, tracheid pits with margo-torus/margo alone; phloem with constitutive ?radial resin canals; Florin Rings in stomata usu. 0; shoots abscise [= cladoptosis]; (leaves opposite - Metasequoia [M.]); ovuliferous cone initially erect; bract scales (opposite - M.), ovuliferous scale invisible; ovules 1-10/bract scale, in two (three - Sequoiadendron) series, adaxial thickening developing above ovules after pollination; pollination droplets coalescing; ovuliferous scales apparent after pollination; (early embryo lacking free-nuclear stage -Sequoia); nuclear genome [1 C] 9.5-10.5(-29 - Sequoia) pg.
3/3: China, Pacific North America.
Age. Crown group Sequoioideae are some (70.2-)60.4(-55.2) Ma (Leslie et al. 2018), (125.0-)93.9(-55.3) Ma (X.-Q. Liu et al. 2022) or ca 104.1 Ma (Fu et al. 2023).
Cognato and Grimaldi (2009) described a scolytine bark beetle = weevil, Microborus inertus, extant species being from Africa and America, in ca 100 Ma amber perhaps from Metasequoia from Myanmar, although Microborus probably infested angiosperms.
[Taxodioideae [Actinostroboideae + Cupressoideae]]: ovules axillary to cone [= bract] scale, straight at maturity, seed not dispersed with part of its supporting structure.
Age. This clade is around 104 Ma (Crisp et al. 2011b), (143-)120(-101) Ma (Leslie et al. 2018) or some (198.5-)171.3(-144.1) Ma (X.-Q. Liu et al. 2022).
5. Taxodioideae K. Koch —— Synonymy: Cryptomeriaceae Gorozhankin, Taxodiaceae Saporta
(Plant deciduous); iso/chamaecydin + [terpenoid]; tracheid pits with margo + torus/margo alone, (phloem with inducible axial resin canals); shoots ultimately abscise [cladoptosis]; ovuliferous cone initially pendulous (horizontal), (bract-scale complex peltate - Taxodium [T.]), ovuliferous scale late-developing, conspicuous after pollination, ovules 1-2(-5)/cone scale; (seed not winged - T.); nuclear genome [1 C] 9-10.5 pg.
3/5: Taxodium (3). East Asia, E. North America, Mexico.
Age. Crown-group Taxodioideae are (101.7-)83.7(-69.0) Ma (Leslie et al. 2018) or (129.7-)99.1(-73.4) Ma (X.-Q. Liu et al. 2022).
It has been suggested that the resin that formed the ca 98.8 Ma amber deposits in Myanmar that are so rich in fossils was produced by Taxodiaceae (Cognato & Grimaldi 2009).
[Actinostroboideae + Cupressoideae]: tracheid pits with margo + torus/(margo alone); branchlets flattened, leaves opposite [not in some seedlings], scale-like, dimorphic (reversal to linear-acicular); fertile cone bract scales whorled, ovuliferous scale 0.
Age. This clade is perhaps 101 Ma (Crisp et al. 2011b), (163-)148.4, 127(-105) Ma (Crisp et al. 2018), (183-)153(-124) or (193.2-)178.2-143.0(-134.3) Ma (Mao et al. 2012), (101.7-)83.7(-69.0) Ma (Leslie et al. 2018) and (175.7-)149.8(-125.0) Ma in X.-Q. Liu et al. (2022); see Z.-Y. Yang et al. (2012) for more dates. This clade is also estimated to be some 153.6 Ma (J. Li et al. 2021) or (154.4-)129.0(-103.7) Ma (X.-Q. Liu et al. 2022).
Map: see Florin (1963, 1966) and Farjon (2005c). Photos - Collection.
6. Actinostroboideae Koehne (= Callitridoideae Saxton) —— Synonymy: Actinostrobaceae Lotsy, Callitridaceae Seward, Diselmaceae A. V. Bobrov & Melikian, Fitzroyaceae A. V. Bobrov & Melikian, Libocedraceae Doweld, Neocallitropsidaceae Doweld, Pilgerodendraceae A. V. Bobrov & Melikian, Widdringtoniaceae Doweld
(Stomata lacking Florin rings); ovuliferous cone erect to pendulous, fertile cone scales 2 whorls, (central columella +); ovules 1-2(-3)/scale, (in two rows).
10/32: Callitris (15). Southern Hemisphere, esp. Chile/Argentina and Australia/New Caledonia, Widdringtonia southern Africa, Papuacedrus New Guinea + Moluccas.
Age. The crown-group age of Actinostroboideae may be a little over 115 Ma (Crisp et al. 2018), around 98 Ma (Crisp et al. 2011b, see also Larter et al. 2017), (102.1-)82.0(-62.5) Ma (Leslie et al. 2018: note topology), 71.9-51.9 Ma (Leslie et al. 2012: other estimates older) or (122.9-)99.5(-76.3) Ma (X.-Q. Liu et al. 2022).
7. Cupressoideae Sweet —— Synonymy: Arceuthidaceae A. V. Bobrov & Melikian, Juniperaceae Berchtold & J. Presl, Limnopityaceae Hayata, Microbiotaceae Nakai, Platycladaceae A. V. Bobrov & Melikian, Tetraclinaceae Hayata, Thujaceae Burnett, Thujopsidaceae Bessey
((Iso)chamaecyadin +), (syringyl lignin + - Tetraclinis); phloem with inducible axial resin canals (resin canals in vascular tissue 0 - Juniperus, Cupressus); nodes 1:2; leaves scale-like to linear; (plant dioecious - Juniperus [J.]); pollen cones often on elongated axes; (1-)2-10(-14) microsporangia/microsporophyll; fertile cone scales 2-6 whorls, (fleshy, fertile and sterile bract-scale complexes - J.), (central columella 0), (bract-scale complex peltate); ovules 1-9(-many)/scale, in 1-6 rows (ovules 12< scale), (ovule 1/cone, terminal or not - Microbiota); (cone fleshy, not opening); (seed takes 2 (embryo dormancy) years to develop - some J., Chamaecyparis), seeds (not winged); cotyledons (-9(-15)), nuclear genome [1C] 9-14(-38 - J.) pg.
13/131: Juniperus (67), Cupressus (12). Esp. Northern Hemisphere, also N.E. Africa.
Age. The crown-group age of this clade is around 129.0 Ma (X.-Q. Liu et al. 2022).
Evolution: Divergence & Distribution. For the Middle Jurassic ca 173 Ma fossil Scitistrobus duncaanensis, see Spencer et al. (2015); it has some similarities with Voltziales, e.g. the ovules are attached to to the free tips of the ovuliferous scale, but in the compact cone, etc., it is assignable to Cupressaceae, and that is where Spencer et al. (2015) place it. There is a rich Jurassic and Cretaceous fossil record of cupressoid plants like Elatides and Hughmillerites that have ovuliferous scales and spiral cone scales, plesiomorphic features, and several of these fossils may form a clade with extant Cunninghamia (sister to all other Cupressaceae), even if, as might be expected with fossil material, support is not overwhelming (Shi et al. 2014). The fossil record of such plants from the northern hemisphere and stretching back to the Jurassic or perhaps even earlier (Rothwell et al. 2011) suggests that they formed an important early radiation in Cupressaceae (e.g. Shi et al. 2014; Atkinson et al. 2014). Herrera et al. (2016) review fossils that can be linked to Cunninghamia and Taiwania, basal in Cupressaceae; see also above. Leslie et al. (2018) provide many dates for clades throughout the family, although relationships may differ from those in Y. Yang et al. (2022).
The distribution of extant Cupressoideae, the old Cupressaceae s. str., is predominantly northern and that of Actinostroboideae is predominantly southern, a vicariance pattern that may reflect the break-up of Pangaea and the formation of Laurasia and Gondwana (Mao et al. 2012; X.-Q. Wang & Ran 2014 for a summary; X.-Q. Liu et al. 2022). The split between the two happened around (183-)153(-124) Ma or (193.2-)178.2-143.0(-134.3) Ma (Mao et al. 2012; see Z.-Y. Yang et al. 2012 and above for more dates). However, there has been much E.-W. and some N.-S. movement even of extant genera - indeed, a number of extant genera are at least Cretaceous if not Jurassic in age. Thus the northern Sequoioideae are repored from rocks from Upper Cretaceous rocks of Queensland (Peters & Christophel 1978: its leaves are like those of the southern Athrotaxis). The southern Actinostroboideae (they were called Callitroideae) seem to have been particularly peripatetic. Here Widdringtonia, now South African, is found in ca 97 Ma rocks from North America (McIver 2001) - an estimate of the stem-group age of Widdringtonia is ca 94 Ma (Crisp et al. 2011b). The largely New Guinean Papuacedrus is known (as P. prechilensis) from Eocene deposits in Argentinian Patagonia some 51.9 and 47.5 Ma, and also from Antarctica, New Zealand, and southern Australia (Wilf et al. 2009); see also Hill and Whang (1996) for Fitzroya from Oligocene Tasmania, Kooyman et al. (2014) and Crisp et al. (2018: this and other genera). The stem-group age of Libocedrus, now known only from New Zealand and New Caledonia and with fossils only from the Antipodes, has been estimated to be (97-)70(-63) Ma, suggesting that it could have been on Zealandia as it broke off from Pangaea, and that both New Zealand and New Caledonia have been long-emergent (Wallis & Jorge 2018); the reference there is to Crisp et al. (2011b), but that would seem to be apocryphal, and the stem-group age of Libocedrus (inc. Pilgerodendron) is given as ca 95 Ma by Crisp et al. (2011b). Wilf and Escapa (2014) suggest some fossil-based dates within this clade. Although Mao et al. (2012) emphasized the importance of vicariance and the break-up of Gondwana in explaining many distribution patterns in Cupressaceae, expecially in Actinostroboideae, increased sampling, etc., suggests that some of these patterns have certainly been facilitated by dispersal events especially around the bottom end of the globe (Crisp et al. 2018). Indeed, extant Actinostroboideae are now very largely plants of the Southern Hemisphere (see also Mothofagaceae for further discussion about such distributions).
In Actinostroboideae in particular there was much Caenozoic extinction, probably around the Oligocene-Miocene boundary ca 23 Ma, and diversification in extant genera can be dated to after this period (Crisp & Cook 2011; Mao et al. 2012; Pittermann et al. 2012); four of the five genera growing in Australia at the beginning of the Oligocene went extinct (Crisp et al. 2018). Arid-adapted members of Actinostroboideae diversified some 52.6-34 Ma (Pittermann et al. 2012); Leslie et al. (2012) suggest that diversification began a little before the K/T boundary ca 65.5 Ma and continued throughout the Caenozoic. Callitris, the most conspicuous element in Australian conifer diversification, is known from both Australia (where it is sometimes a dominant tree) and New Caledonia; it can handle water loss well (see below), and it may grow along with Eucalyptus which has a very different water management system (see also Larter et al. 2017; Lawes & Neumann 2022). Here initial diversification seems to have happened rapidly (short branch lengths) around 30 Ma and there was a subsequent round of diversification in the last 17 My as extreme aridity developed in Central Australia, the Pleistocene glacial-interglacial cycling also affecting the plants (Larter et al. 2017; M. A. M. Renner et al. 2019). Overall, Actinostroboideae appear to have originated in Australia—New Guinea and then moved to South America (X.-Q. Liu et al. 2022). Within Cupressoideae, Pittermann et al. (2012; see also Edwards & Donoghue 2013) suggested that Juniperus and Cupressus s.l., most of whose species have become adapted to dry conditions and have developed cavitation-resistant xylem, diverged 38.7-32 Ma, while figures in Leslie et al. (2012) are 53-33 Ma (and some much older). Mao et al. (2010; see also Adams & Schwarzbach 2013) throught that there was E->W migration across the North Atlantic Land Bridge in Juniperus, initially Eurasian in distribution, around 47-30.3 Ma (Mao et al. 2010 for more dates). On the other hand, Liu et al. 2022) suggested that Cupressoideae were Asian in origin, and that there were three Asian—North American disjunctions within the subfamily.
X.-Q. Liu et al. 2022) suggest that phylogenetic analyses in the future should cater for reticulate evolution (see Genes & Genomes below).
Ecology & Physiology. Biomass estimates for forests with Sequoia sempervirens are 2.3 x 106 kg ha-1 (Franklin & Dryness 1973).
Callitris is perhaps one of the most stress-resistant plants in the world although some species grow in quite mesic conditions (Brodribb et al. 2013b). It is a fire-adapted, shallow-rooted tree with remarkable foliar anatomy, that is, it has scale leaves developing and becoming more or less adnate to and covering the stem like the phyllichnia (= modified leaf whorls) of Casuarina (Dörken et al. 2020). Hydraulic tension in the stem can rise to >8 MPa before significant stem cavitation occurs (rather like Juniperus ), growth is very sensitive to rainfall, its stomata close only slowly as the soil dries out (the plants are anisohydric), etc. (Brodribb et al. 2013b). See also Larter et al. (2017) for the behaviour of this remarkable plant in response to drying out, etc. - the shallow rooting system, extremely cavitation-resistant xylem, and the low stomatal sensitivity to dessication were all particularly important - indeed, they noted that the Callitris clade included the most embolism-resistant species known, and that this extreme embolism resistance had evolved more than once here. Moreover, "no significant decrease in xylem specific hydraulic conductance with increasing aridity and increasing resistance to embolism" was detected here (ibid. p. 109).
Pittermann et al. (2010) discussed details of xylem function in relation to the environment in Cupressaceae; the ancestors of Cupressaceae may have had low cavitation resistance. The initial preferences of the family were for mesic conditions, but Pittermann et al. (2012) noted that a number of species of both Cupressoideae and Actinostroboideae had evolved drought resistance, their xylem-specific conductivity and stomatal conductances being lower, and also their CO2 assimilation rates were much reduced. The cost of these adaptations was slow growth. Metasequoia glyptostroboides, on the other hand, may grow in wet conditions and its transcriptional response to flooding stress is similar to that of angiosperms (Fu et al. 2023). Interestingly, the niche (Grinellian) of M. glyptostroboideds has remained largely constant over time (Quirk et al. 2024).
Resin from various Cupressaceae has formed amber in several parts of the world since the Late Cretaceous (Seyfullah et al. 2018).
Pollination Biology & Seed Dispersal. For the active and irreversible withdrawal of pollination drops within half an hour of deposition of pollen, see Dörken and Jagel (2014); this also happens when there is pollen from other Cupressaceae in the drops, but not when there is pollen from angiosperms or Pinus. In Cupressus the pollination droplets of adjacent ovules may become confluent (Jagel & Stützel 2001), a sort of gymnospermous extragynoecial compitum.
For monoecy, dioecy, etc., see Jagel and Dörken (2015a). There is paternal apomixis in Cupressus dupreziana, unknown in any other seed plant. Here the embryo develops from unreduced male gametes (Pichot et al. 2000, 2001), but there are other forms of androgenesis, asexual reproduction of the male nuclear genome, as in Solanaceae, etc. (Hedtke & Hillis 2011).
For details of seed morphology, dispersal, etc., and their evolution, see Contreras et al. (2016); dry and winged (the wings develop from the ovules) seeds are common, but Juniperus is noted for its fleshy disseminules. Serotiny has evolved five times here, and it is associated with the evolution of larger seed cones (Swarup et al. 2020).
Plant-Bacterial/Fungal Associations. The telial stage of Gymnosporangium rust is common on some Cupressaceae, especially Juniperus, while the aecial stage characterises Rosaceae-Maloideae (Savile 1979b).
Genes & Genomes. Sequoia sempervirens has a large nuclear genome (26.5 Gb) compared with other members of this clade; the species is an autopolyploid (Fu et al. 2023). See Zonneveld (2014) for discussion of genome size in taxa like Juniperus; there are connections with polyploidy, although polyploidy is rather uncommon in Pinales - but see Ephedra (Gnetales) in particular. There seems to have been an ancient hybridization event within Cupressoideae, i.e. between the ancestors of the clades [Microbiota, Platycladus, Tetraclinis] and [Juniperus, Cupressus, Hesperocyparis, Callitropsis, Xanthocyparis] (X.-Q. Liu et al. 2022; Stull et al. 2023); the crown-group age of the latter clade is estimated to be around 62 Ma (Liu et al. 2022). The ycf1/2 area discussed below is evidence of a very ancient introgression event some 60-40 Ma (A. Zhu et al. 2018).
Cryptomeria japonica has a much reduced chloroplast inverted repeat that includes only a few genes (Hirao et al. 2008), and the loss of this repeat in Cupressaceae is connected with the fact that the plastome is often small, as in Callitris where it is only about 121 kb (C.-S. Wu & Chaw 2016). For the organization of the plastome throughout the family, see Wu and Chaw (2016) and Qu et al. (2017) and references. Wu and Chaw (2016) noted the frequency of plastome inversions in this clade - there are some 28 - compared to how uncommon they are in other conifers - Araucariaceae, for example have none, Taxaceae and Podocarpaceae only one. Isomeric plastomes are known from Cupressaceae, substoichiometric shifting occuring - there is recombination at repeats (Qu et al. 2017).
Mitochondria here have lost 8-9 genes and 11-12 introns (Guo et al. 2020).
Chemistry, Morphology, etc.. Characters of wood anatomy may yield phylogenetically interesting variation (Schulz & Stützel 2007), but state delimitation is difficult; for epidermal morphology, see Q.-W. Ma et al. (2009).
Proliferation of the ovuliferous cone is common, and the distribution of this feature may be of phylogenetic interest (Schulz & Stützel 2007). Scales on the ovuliferous cones are wedge-shaped to peltate, but most Cupressaceae lack ovuliferous scales, having only bract (= cone) scales (Zhang et al. 2004; see also Farjon 2005c; Jagel & Dörken 2015a), however, Cryptomeria has several "teeth" on the ovuliferous scale and some other subfamilies have structures that appear to be much reduced ovuliferous scales (see also Schulz & Stützel 2007). However, the ovuliferous scales and ovules may also be interpreted as the products of serial buds in the axils of the bract scales, the oldest buds being furthest away from the bract scale, rather as are sometimes found in the vegetative part of the plant (Dörken & Rudall 2018: note interpretation of epidermal layer surrounding the ovule in terata). In some Cupressoideae the ovules are not axillary, indeed, the variation in ovule number and position within the cone is considerable (Schulz et al. 2003; Jagel & Dörken 2015a). There is substantial variation in how the cone develops, the seeds being enclosed in various ways (e.g. Jagel & Dörken 2014; Dörken & Rudall 2018) and varying considerably in both their numbers and positional relationships to the bract scales (e.g. Jagel & Dörken 2015a). Fertile and sterile bract-scale complexes make up the fleshy cone of Juniperus (Nigris et al. 2021).
For general information, see Page (1990: Cupressaceae, Taxodiaceae) and for a monograph (and far more) of Cupressaceae see Farjon (2005c); general information can be found in the Gymnosperm Database and see Contreras et al. (2019) for information about basal taxa, both living and dead. For some terpenoids, see Otto et al. (2002), for Florin Rings in stomata, see Oladele (1983), for cone and ovule morphology, see e.g. Lemoine-Sébastian (1968a, b, esp. Juniperus-Junipereae), Takaso and Tomlinson (1989, 1990, 1992), Jagel and Stützel (2001), Farjon and Garcia (2003) and Jagel and Dörken (2015b) and for embryogeny, see Chowdhury (1963), J. Doyle (1963: survey of early embryogeny, little known in Callitroideae) and Dogra (1967: Taxodioideae).
Phylogeny. Page (1990) suggested that there were "fundamental" differences between Cupressaceae and Taxodiaceae in the morphology of their reproductive parts, but in the tree of Quinn et al. (2002) Cupressaceae s. str. were embedded in a paraphyletic Taxodiaceae which formed a basal grade. Phenetic analyses had earlier suggested the combination of the two (Eckenwalder 1976), and they are combined in Farjon (2005c), and the broad circumscription of the family is used here. For early papers dealing with relationships within Cupressaceae, see Brunsfeld et al. (1994), Gadek et al. (2000), Kusumi et al. (2000), Farjon et al. (2002), Brunsfeld et al. (2003), and Little et al. (2004). The basic phylogenetic structure of the family is probably [Cunninghamioideae [Taiwanioideae [Athrotaxidoideae [Sequoioideae [Taxodioideae [Cupressoideae + Callitridoideae (= Actinostroboideae)]]]]]] (Mao et al. 2012: much support strong; Sen et al. 2016; Z.-D. Chen et al. 2016: no Athrotaxis), but Z.-Y. Yang et al. (2012) found the subfamilial order in the middle of the tree to be [... [Sequoioideae [Athrotaxidoideae [...]]]] while Y. Lu et al. (2014) and others have recovered a clade [Athrotaxidoideae + Sequoioideae] (recognized above), however, other major groupings were similar. Thus the phylogeny is usually strongly pectinate basally, although in some morphological analyses two or more members of the first three branches may unite to form a single clade (e.g. Rothwell et al. 2011). Indeed, recent work by X.-Q. Liu et al. (2022: all 32 genera, 46 spp., 1944 orthogroups) suggests that this pectination should be modified, and instead of ...[Taiwanioideae [Athrotaxidoideae [Sequoioideae [Taxodioideae... relationships are ...[Taiwanioideae [[Athrotaxidoideae + Sequoioideae] [Taxodioideae... (see also Leebens-Mack et al. 2019; Stull et al. 2021), and this topology is followed above, although Stull et al. (2021) found little support for the position of Athrotaxidoideae.
Within Actinostroboideae/Callitoideae, Callitris was found to be paraphyletic, although morphological (Piggin & Bruhl 2010) and molecular (Pye et al. 2003) studies do not agree over the extent of the paraphyly (see also e.g. Crisp et al. 2018). Papuacedrus is probably sister to the rest of the subfamily, possibly followed by Austrocedrus, although the position of the latter is uncertain (Crisp et al. 2018; see also Y. Lu et al. 2014;; Leslie et al. 2018), while Larter et al. (2017: 5 plastid and 3 nuclear markers) found that Actinostrobus and the New Caledonian Neocallitropsis were embedded in Callitris, other relationships being [Papuacedrus [[Pilgerodendron + Libocedrus] [[Widdringtonia [Diselma + Fitzroya]] Callitris s.l.]]. Y. Yang et al. (2022) recovered a clade [Papuacedrus + Austrocedrus] that was part of a basal tritomy in the subfamily.
Within Cupressoideae, Lu et al. (2014; see also Y. Yang et al. 2022) found that the clade [Thuja + Thujopsis] was sister to the rest of the subfamily while in J. Li et al. (2021: check) outline relationships as being [Papuacedrus [Thujopsis + Cupressus]]. For relationships in Juniperus, see Mao et al. (2010) and Adams and Schwarzbach (2013). Cupressus has turned out to be polyphyletic and is now restricted to the Old World (Xiang & Li 2005; especially Little 2006); for the limits of Cupressus and Chamaecyparis, see also Jagel and Stützel (2001). Terry and Adams (2015: sampling slight) suggest relationships in this area are [Cupressus [Juniperus [Hesperocyparis, Callitropsis, Xanthocyparis]]] (see also Y. Lu et al. 2014; Mao et al. 2018), although chloroplast data were a bit wayward. Indeed, A. Zhu et al. (2018) found that a ca 15 kb part of the plastome, the ycf1/2 area, helped to produce the conflicting relationships that have been obtained around here (e.g. Farjon et al. 2002; Little et al. 2004; Mao et al. 2010; Terry & Adams 2015 and references), inclusion of that segment in plastome analyses giving a [Juniperus + Cupressus] clade, while in other analyses the clade [Cupressus [Xanthocyparis [Callitropsis + Hesperocyparis]]] was recovered. Leslie et al. (2018) and Y. Yang et al. (2022) found that a clade [Thuja + Thujopsis] was well supported as sister to the rest of the subfamily. For relationships in Juniperus and other genera, see Leslie et al. (2018).
Classification. Having 24 family names for the ca 30 genera in this one family says a lot about the past. For generic limits around Cupressus, which has turned out to be polyphyletic, see Price and Adams (2009), Little (2006) and A. Zhu et al. (2018), for those around Callitris, see Pye et al. (2003) and Piggin and Bruhl (2010), and for the recognition of the sections of Juniperus as separate genera, see Y. Yang et al. (2022). For an account of Cupressus, see Adams (2010).
Botanical Trivia. Juniperus (Cupressoideae) grows at some 4,900 m altitude on the Tibetan Plateau and forms the highest known forest (Opganoorth et al. 2010), but c.f. the Andean Polylepis (Rosaceae).
The tallest living tree in the world is a coastal redwood Sequoia sempervirens (Sequoioideae), at about 115.5 metres (379 feet), although the giant redwood (Sequoiadendron giganteum) is larger in terms of biomass and Eucalyptus regnans was almost certainly taller. Sequoia also has the longest fusiform initials known, ca 9 mm long, over five times he length of fusiform initials in angiosperms (Philipson et al. 1961).
Fitzroya cupressoides (Actinostroboideae) is the largest tree in South America, over 70 m tall and with a d.b.h ca 12.6 m (before there was much logging, now only about a third of this), and the oldest individual, at 3,622 years, is the second oldest seed plant known (Lara & Villalba 1993).